Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma
- PMID: 30097565
- PMCID: PMC6086830
- DOI: 10.1038/s41467-018-05681-9
Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma
Erratum in
-
Author Correction: Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma.Nat Commun. 2018 Sep 20;9(1):3914. doi: 10.1038/s41467-018-06428-2. Nat Commun. 2018. PMID: 30237502 Free PMC article.
Abstract
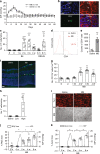
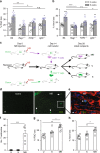
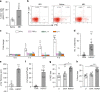
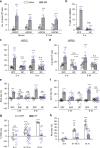
Glaucoma is the most prevalent neurodegenerative disease and a leading cause of blindness worldwide. The mechanisms causing glaucomatous neurodegeneration are not fully understood. Here we show, using mice deficient in T and/or B cells and adoptive cell transfer, that transient elevation of intraocular pressure (IOP) is sufficient to induce T-cell infiltration into the retina. This T-cell infiltration leads to a prolonged phase of retinal ganglion cell degeneration that persists after IOP returns to a normal level. Heat shock proteins (HSP) are identified as target antigens of T-cell responses in glaucomatous mice and human glaucoma patients. Furthermore, retina-infiltrating T cells cross-react with human and bacterial HSPs; mice raised in the absence of commensal microflora do not develop glaucomatous T-cell responses or the associated neurodegeneration. These results provide compelling evidence that glaucomatous neurodegeneration is mediated in part by T cells that are pre-sensitized by exposure to commensal microflora.
Conflict of interest statement
H.C., K.-S.C., D.F.C., and J.C. are co-inventors on a pending patent application for targeting T cells and microbiome for optic neuropathy therapy. The other authors declare no competing interests.
Figures


Comment in
-
Bacteria-primed T cells identified as culprit in glaucoma.Nat Rev Immunol. 2018 Oct;18(10):603. doi: 10.1038/s41577-018-0063-x. Nat Rev Immunol. 2018. PMID: 30185929 No abstract available.
References
-
- McKinnon SJ, Goldberg LD, Peeples P, Walt JG, Bramley TJ. Current management of glaucoma and the need for complete therapy. Am. J. Manag. Care. 2008;14:S20–S27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R41 EY025913/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)/International
- R01 EY025259/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)/International
- R41 EY025913/EY/NEI NIH HHS/United States
- P30 CA014051/CA/NCI NIH HHS/United States
- R01 AI069208/AI/NIAID NIH HHS/United States
- R01 EY025259/EY/NEI NIH HHS/United States
- R01 NS038253/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)/International
- R21 EY027067/EY/NEI NIH HHS/United States
- R01 NS038253/NS/NINDS NIH HHS/United States
- U54 HD090255/HD/NICHD NIH HHS/United States
- R21 EY027067/U.S. Department of Health & Human Services | NIH | National Eye Institute (NEI)/International
- P30 EY003790/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

