P2X7 as a scavenger receptor for innate phagocytosis in the brain
- PMID: 30098011
- PMCID: PMC6193880
- DOI: 10.1111/bph.14470
P2X7 as a scavenger receptor for innate phagocytosis in the brain
Abstract
The P2X7 receptor has been widely studied for its ATP-induced pro-inflammatory effect, but in the absence of a ligand, P2X7 has a second function as a scavenger receptor, which is active in the development of the human brain. The scavenger activity of P2X7 is only evident in the absence of serum but is fully active in cerebrospinal fluid. P2X7 on the cell surface is present as a membrane complex, and an attachment to non-muscle myosin of the cytoskeleton is required for particle engulfment. Selective antagonists of P2X7 pro-inflammatory function have little effect on phagocytosis, but inheritance of a variant haplotype spanning the P2RX7 and P2RX4 genes has been associated with loss of P2X7-mediated phagocytosis. Recent studies in mice suggest that the innate phagocytosis mediated by P2X7 receptors declines with ageing. Thus, defective P2X7-mediated phagocytosis may contribute to age-related neuro-degenerative diseases including Alzheimer's disease, age-related macular degeneration and primary progressive multiple sclerosis.
© 2018 The British Pharmacological Society.
Figures
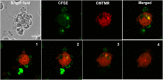
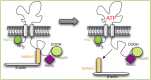
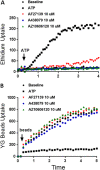
References
-
- Adinolfi E, Kim M, Young MT, Di Virgilio F, Surprenant A (2003). Tyrosine phosphorylation of HSP90 within the P2X7 receptor complex negatively regulates P2X7 receptors. J Biol Chem 278: 37344–37351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

