Enteral nutrition for maintenance of remission in Crohn's disease
- PMID: 30098021
- PMCID: PMC6513617
- DOI: 10.1002/14651858.CD005984.pub3
Enteral nutrition for maintenance of remission in Crohn's disease
Abstract
Background: Prevention of relapse is a major issue in the management of quiescent Crohn's disease (CD). Current therapies (e.g. methotrexate, biologics, 6-mercaptopurine and azathioprine) may be effective for maintaining remission in CD, but these drugs may cause significant adverse events. Interventions that are effective and safe for maintenance of remission in CD are desirable.
Objectives: The primary objectives were to evaluate the efficacy and safety of enteral nutrition for the maintenance of remission in CD and to assess the impact of formula composition on effectiveness.
Search methods: We searched MEDLINE, Embase, CENTRAL, the Cochrane IBD Group Specialized Register and clinicaltrials.gov from inception to 27 July 2018. We also searched references of retrieved studies and reviews.
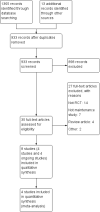
Selection criteria: Randomised controlled trials (RCTs) including participants of any age with quiescent CD were considered for inclusion. Studies that compared enteral nutrition with no intervention, placebo or any other intervention were selected for review.
Data collection and analysis: Two authors independently screened studies for inclusion, extracted data and assessed methodological quality using the Cochrane risk of bias tool. The primary outcome was clinical or endoscopic relapse as defined by the primary studies. Secondary outcomes included anthropometric measures (i.e. height and weight), quality of life (QoL), adverse events, serious adverse events and withdrawal due to adverse events. We calculated the risk ratio and 95% confidence interval (CI) for dichotomous outcomes. For continuous outcomes, we calculated the mean difference and 95% CI. A random-effects model was used for the statistical analysis. We used the GRADE criteria to assess the overall certainty of the evidence supporting the primary outcome and selected secondary outcomes.
Main results: Four RCTs (262 adult participants) met the inclusion criteria. One study (N = 33) compared an elemental diet to a non-elemental (polymeric) diet. One study (N = 51) compared a half elemental diet to a regular free diet. Another study (N = 95) compared an elemental diet to 6-mercaptopurine (6-MP) or a no treatment control group. One study (N= 83) compared a polymeric diet to mesalamine. Two studies were rated as high risk of bias due to lack of blinding or incomplete outcome data. The other two studies were judged to have an unclear risk of bias. The studies were not pooled due to differences in control interventions and the way outcomes were assessed.The effect of an elemental diet compared to a polymeric diet on remission rates or withdrawal due to adverse events is uncertain. Fifty-eight per cent (11/19) of participants in the elemental diet group relapsed at 12 months compared to 57% (8/14) of participants in the polymeric diet group (RR 1.01, 95% CI 0.56 to 1.84; very low certainty evidence). Thirty-two per cent (6/19) of participants in the elemental diet group were intolerant to the enteral nutritional formula because of taste or smell and were withdrawn from the study in the first 2 weeks compared to zero participants (0/14) in the polymeric diet group (RR 9.75, 95% CI 0.59 to 159.93; low certainty evidence). Anthropometric measures, QoL, adverse events and serious adverse events were not reported as outcomes.The effect of an elemental diet (half of total daily calorie requirements) compared to a normal free diet on relapse rates is uncertain. Thirty-five per cent (9/26) of participants in the elemental diet group relapsed at 12 months compared to 64% (16/25) of participants in the free diet group (RR 0.54, 95% CI 0.30 to 0.99; very low certainty evidence). No adverse events were reported. This study reported no differences in weight change between the two diet groups. Height and QoL were not reported as outcomes.The effect of an elemental diet compared to 6-MP on relapse rates or adverse events is uncertain. Thirty-eight per cent (12/32) of participants in the elemental diet group relapsed at 12 months compared to 23% (7/30) of participants in the 6-MP group (RR 1.61; 95% CI 0.73 to 3.53; very low certainty evidence). Three per cent (1/32) of participants in the elemental diet group had an adverse event compared to 13% (4/30) of participants in the 6-MP group (RR 0.23, 95% CI 0.03 to 1.98; low certainty evidence). Adverse events in the elemental diet group included surgery due to worsening CD. Adverse events in the 6-MP group included liver injury (n = 2), hair loss (n = 1) and surgery due to an abscess (n = 1). No serious adverse events or withdrawals due to adverse events were reported. Weight, height and QoL were not reported as outcomesThe effect of a polymeric diet compared to mesalamine on relapse rates and weight is uncertain. Forty-two per cent (18/43) of participants in the polymeric diet group relapsed at 6 months compared to 55% (22/40) of participants in the mesalamine group (RR 0.76; 95% CI 0.49 to 1.19; low certainty evidence). The mean difference in weight gain over the study period was 1.9 kg higher in the polymeric diet group compared to mesalamine (95% CI -4.62 to 8.42; low certainty evidence). Two participants in the polymeric diet group experienced nausea and four had diarrhoea. It is unclear if any participants in the mesalamine group had an adverse event. Height, QoL, serious adverse events and withdrawal due to adverse events were not reported as outcomes.
Authors' conclusions: The results for the outcomes assessed in this review are uncertain and no firm conclusions regarding the efficacy and safety of enteral nutrition in quiescent CD can be drawn. More research is needed to determine the efficacy and safety of using enteral nutrition as maintenance therapy in CD. Currently, there are four ongoing studies (estimated enrolment of 280 participants). This review will be updated when the results of these studies are available.
Conflict of interest statement
Anthony K Akobeng: None known
Dongni Zhang: None known
Morris Gordon has received travel fees to attend international scientific and training meeting such as DDW, Advances in IBD, ESPGHAN, BSPGHAN and Cochrane focused international events from companies including: Abbott, Nutricia, Biogaia, Ferring, Allergan, Tillotts. None of these companies have had any involvement in any works completed by me and I have never had any payments for any other activates from these companies.
John K MacDonald: None known
Figures
Update of
-
Enteral nutrition for maintenance of remission in Crohn's disease.Cochrane Database Syst Rev. 2007 Jul 18;(3):CD005984. doi: 10.1002/14651858.CD005984.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2018 Aug 11;8:CD005984. doi: 10.1002/14651858.CD005984.pub3. PMID: 17636816 Updated.
References
References to studies included in this review
Hanai 2012 {published data only}
-
- Hanai H, Iida T, Takeuchi K, Arai H, Arai O, Abe J, et al. Nutritional therapy versus 6‐mercaptopurine as maintenance therapy in patients with Crohn's disease. Digestive and Liver Disease 2012;44(8):649‐54. - PubMed
Takagi 2006 {published data only}
-
- Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M, et al. Effectiveness of an 'half elemental diet' as maintenance therapy for Crohn's disease: A randomized‐controlled trial. Alimentary Pharmacology & Therapeutics 2006;24(9):1333‐40. - PubMed
Triantafillidis 2010 {published data only}
-
- Triantafillidis JK, Stamataki A, Karagianni V, Gikas A, Malgarinos G. Maintenance treatment of Crohn's disease with a polymeric feed rich in TGF‐beta. Annals of Gastroenterology 2010;23(2):113‐8.
Verma 2001 {published data only}
-
- Verma S, Holdsworth CD, Giaffer MH. Does adjuvant nutritional support diminish steroid dependency in Crohn disease?. Scandinavian Journal of Gastroenterology 2001;36(4):383‐8. - PubMed
References to studies excluded from this review
Canani 2006 {published data only}
-
- Canani RB, Terrin G, Borrelli O, Romano MT, Manguso F, Coruzzo A, et al. Short‐ and long‐term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn's disease. Digestive and Liver Disease 2006;38(6):381‐7. - PubMed
Dray 2005 {published data only}
-
- Dray X, Marteau P. The use of enteral nutrition in the management of Crohn's disease in adults. JPEN. Journal of Parenteral and Enteral Nutrition 2005;29(4 Suppl):S166‐72. - PubMed
Esaki 2005 {published data only}
-
- Esaki M, Matsumoto T, Hizawa K, Nakamura S, Jo Y, Mibu R, et al. Preventive effect of nutritional therapy against postoperative recurrence of Crohn disease, with reference to findings determined by intra‐operative enteroscopy. Scandinavian Journal of Gastroenterology 2005;40(12):1431‐7. - PubMed
Forbes 2002 {published data only}
-
- Forbes A. Review article: Crohn's disease‐‐the role of nutritional therapy. Alimentary Pharmacology & Therapeutics 2002;16(Suppl 4):48‐52. - PubMed
Giaffer 1991 {published data only}
-
- Giaffer MH, Cann P, Holdsworth CD. Long‐term effects of elemental and exclusion diets for Crohn's disease. Alimentary Pharmacology & Therapeutics 1991;5(2):115‐25. - PubMed
Gorard 1993 {published data only}
Griffiths 2005 {published data only}
-
- Griffiths AM. Enteral nutrition in the management of Crohn's disease. JPEN. Journal of Parenteral and Enteral Nutrition 2005;29(4 Suppl):S108‐17. - PubMed
Harries 1983 {published data only}
-
- Harries AD, Jones LA, Danis V, Fifield R, Heatley RV, Newcombe RG, et al. Controlled trial of supplemented oral nutrition in Crohn's disease. Lancet 1983;1(8330):887‐90. - PubMed
Hirakawa 1993 {published data only}
-
- Hirakawa H, Fukuda Y, Tanida N, Hosomi M, Shimoyama T. Home elemental enteral hyperalimentation (HEEH) for the maintenance of remission in patients with Crohn's disease. Gastroenterologia Japonica 1993;28(3):379‐84. - PubMed
Iakovlev 2014 {published data only}
-
- Iakovlev AA, Kazaryan TS. Optimization of diagnosis and treatment of nutritional insufficiency in patients with inflammatory bowel disease. United European Gastroenterology Journal 2014;1:A286.
Johnson 2006 {published data only}
Jones 1987 {published data only}
-
- Jones VA. Comparison of total parenteral nutrition and elemental diet in induction of remission of Crohn's disease. Long‐term maintenance of remission by personalized food exclusion diets. Digestive Diseases and Sciences 1987;32(12 Supplement):100S‐7S. - PubMed
Knight 2005 {published data only}
-
- Knight C, El‐Matary W, Spray C, Sandhu BK. Long‐term outcome of nutritional therapy in paediatric Crohn's disease. Clinical Nutrition (Edinburgh, Scotland) 2005;24(5):775‐9. - PubMed
Koga 1993 {published data only}
-
- Koga H, Iida M, Aoyagi K, Matsui T, Fujishima M. Long‐term efficacy of low residue diet for the maintenance of remission in patients with Crohn's disease. Nippon Shokakibyo Gakkai Zasshi 1993;90(11):2882‐8. - PubMed
Matsueda 1995 {published data only}
-
- Matsueda K, Shoda R, Takazoe M, Hiwatashi N, Bamba T, Kobayashi K, et al. Therapeutic efficacy of cyclic home elemental enteral alimentation in Crohn's disease: Japanese cooperative Crohn's disease study. Journal of Gastroenterology 1995;30(Suppl 8):91‐4. - PubMed
Navarro 1982 {published data only}
-
- Navarro J, Vargas J, Cezard JP, Charritat JL, Polonovski C. Prolonged constant rate elemental enteral nutrition in Crohn's disease. Journal of Pediatric Gastroenterology and Nutrition 1982;1(4):541‐6. - PubMed
Nomura 1995 {published data only}
-
- Nomura M, Taruishi M, Ashida T, Ayabe T, Einami K, Saitoh Y, et al. Home enteral nutrition for the maintenance of remission in patients with Crohn's disease‐‐including comparison between Elental and Enterued. Nippon Shokakibyo Gakkai Zasshi 1995;92(1):32‐40. - PubMed
Phylactos 2001 {published data only}
-
- Phylactos AC, Fasoula IN, Arnaud‐Battandier F, Walker‐Smith JA, Fell JM. Effect of enteral nutrition on antioxidant enzyme systems and inflammation in paediatric Crohn's disease. Acta Paediatrica 2001;90(8):883‐8. - PubMed
Roggero 2003 {published data only}
-
- Roggero P, Santus F, Barabino A, Canani RB, Cucchiara S, Guariso G, et al. A prospective pediatric multicenter trial of enteral nutrition and azathioprine in preventing relapses of Crohn disease: preliminary results. Journal of Pediatric Gastroenterology and Nutrition 2003;36(4):543.
Triantafillidis 2006 {published data only}
-
- Triantafillidis JK, Stamataki A, Gikas A, Sklavaina M, Mylonaki M, Georgopoulos F, et al. Beneficial effect of a polymeric feed, rich in TGF‐beta, on adult patients with active Crohn's disease: A pilot study. Annals of Gastroenterology 2006;19(1):66‐71.
Valentini 2002 {published data only}
-
- Valentini G, Capristo E, Scarfone A, Mingrone G, Greco AV, Gasbarrini G. Enteral diet in Crohn's disease. Nutritional and therapeutic implications in patient's management. Minerva Gastroenterologica e Dietologica 2002;48(1):13‐24. - PubMed
Verma 2000 {published data only}
-
- Verma S, Kirkwood B, Brown S, Giaffer MH. Oral nutritional supplementation is effective in the maintenance of remission in Crohn's disease. Digestive and Liver Disease 2000;32(9):769‐74. - PubMed
Wilschanski 1996 {published data only}
Yamamoto 2005 {published data only}
-
- Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K. Impact of elemental diet on mucosal inflammation in patients with active Crohn's disease: cytokine production and endoscopic and histological findings. Inflammatory Bowel Diseases 2005;11(6):580‐8. - PubMed
Yamamoto 2007 {published data only}
-
- Yamamoto T, Nakahigashi M, Umegae S, Kitagawa T, Matsumoto K. Impact of long‐term enteral nutrition on clinical and endoscopic recurrence after resection for Crohn's disease: A prospective, non‐randomized, parallel, controlled study. Alimentary Pharmacology & Therapeutics 2007;25(1):67‐72. - PubMed
Yamamoto 2010 {published data only}
-
- Yamamoto T, Nakahigashi M, Umegae S, Matsumoto K. Prospective clinical trial: enteral nutrition during maintenance infliximab in Crohn's disease. Journal of Gastroenterology 2010;45(1):24‐29. - PubMed
Yamamoto 2015 {published data only}
-
- Yamamoto T, Nakahigashi M, Shimoyama T, Matsumoto K. The long‐term efficacy of concomitant enteral nutritional therapy during maintenance infliximab in patients with Crohn's disease: A prospective observational trial. Journal of Crohn's and Colitis 2015;9:S364.
References to ongoing studies
NCT01823042 {published data only}
-
- NCT01823042. A Randomized, Controlled, Open‐label Study to Assess the Efficacy of Enteral Nutrition in Fill of the Treatment Blank Period of the Postoperative Maintain Remission Medication for Crohn's Disease (CD). clinicaltrials.gov/show/NCT01823042 (accessed 3 August 2017).
NCT02201693 {published data only}
-
- NCT02201693. Randomised Trial Comparing 12 Months of Cyclic Enteral Nutrition to Supplementary Enteral Nutrition as Maintenance Therapy for Pediatric Crohn's Disease. clinicaltrials.gov/show/NCT02201693 (accessed 3 August 2017).
NCT02231814 {published data only}
-
- NCT02231814. Dietary Therapy Using Partial Enteral Nutrition and the Crohn's Disease Exclusion Diet (CDED) for Induction and Maintenance of Remission in Mild to Moderate Crohn's Disease in Adults‐ A Pilot Study. clinicaltrials.gov/show/NCT02231814 (accessed 3 August 2017).
NCT02843100 {published data only}
-
- NCT02843100. Modified Exclusive Enteral Nutrition With the Crohn's Disease Exclusion Diet for Induction and Maintenance of Remission and Re‐biosis. clinicaltrials.gov/show/NCT02843100 (accessed 3 August 2017).
Additional references
Akobeng 2000
-
- Akobeng AK, Miller V, Stanton J, Elbadri AM, Thomas AG. Double‐blind randomized controlled trial of glutamine‐enriched polymeric diet in the treatment of active Crohn's disease. Journal of Pediatric Gastroenterology and Nutrition 2000;30(1):78‐84. - PubMed
Akobeng 2016
Biancone 2003
-
- Biancone L, Tosti C, Fina D, Fantini M, Nigris F, Geremia A, et al. Review article: maintenance treatment of Crohn's disease. Alimentary Pharmacology & Therapeutics 2003;17(Suppl 2):31‐7. - PubMed
Chande 2015
Colombel 2007
-
- Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology 2007;132(1):52‐65. - PubMed
Egger 1997
Feagan 2000
-
- Feagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. North American Crohn's Study Group Investigators. New England Journal of Medicine 2000;342(22):1627‐32. - PubMed
Feagan 2003
-
- Feagan BG. Maintenance therapy for inflammatory bowel disease. American Journal of Gastroenterology 2003;98 (12 Suppl):S6‐S17. - PubMed
Goh 2003
-
- Goh J, O'Morain CA. Review article: nutrition and adult inflammatory bowel disease. Alimentary Pharmacology & Therapeutics 2003;17(3):307‐20. - PubMed
Gonzalez‐Huix 1993
Guyatt 2008
Hanauer 2002
-
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002;359(9317):1541‐9. - PubMed
Heuschkel 2000
-
- Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral nutrition and corticosteroids in the treatment of acute Crohn's disease in children. Journal of Pediatric Gastroenterology and Nutrition 2000;31(1):8‐15. - PubMed
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jones 1985
-
- Jones VA, Dickinson RJ, Workman E, Wilson AJ, Freeman AH, Hunter JO. Crohn's disease: maintenance of remission by diet. Lancet 1985;2(8448):177‐80. - PubMed
Lewis 1994
-
- Lewis JD, Fisher RL. Nutrition support in inflammatory bowel disease. Medical Clinics of North America 1994;78(6):1443‐56. - PubMed
Lichtenstein 2004
-
- Lichtenstein GR, Yan S, Bala M, Hanauer S. Remission in patients with Crohn's disease is associated with improvement in employment and quality of life and a decrease in hospitalizations and surgeries. American Journal of Gastroenterology 2004;99(1):91‐6. - PubMed
Murphy 2001
-
- Murphy MS, Randell T. Evaluation of elemental diet therapy as a long term strategy for managing Crohn's disease (abstract). Journal of Pediatric Gastroenterology and Nutrition 2001;32:366.
Narula 2018
Patel 2014
Pitkin 1999
-
- Pitkin RM, Branagan MA, Burmeister LF. Accuracy of data in abstracts of published research articles. JAMA 1999;281(12):1110‐1. - PubMed
Sandborn 2013
-
- Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn's disease. New England Journal of Medicine 2013;369(8):711‐21. - PubMed
Schunemann 2011
-
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Steinhart 2003
Thomas 1993
-
- Thomas AG, Taylor F, Miller V. Dietary intake and nutritional treatment in childhood Crohn's disease. Journal of Pediatric Gastroenterology and Nutrition 1993;17(1):75‐81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous