Single- and multiple-dose pharmacokinetics and safety of pimodivir, a novel, non-nucleoside polymerase basic protein 2 subunit inhibitor of the influenza A virus polymerase complex, and interaction with oseltamivir: a Phase 1 open-label study in healthy volunteers
- PMID: 30098042
- PMCID: PMC6177716
- DOI: 10.1111/bcp.13733
Single- and multiple-dose pharmacokinetics and safety of pimodivir, a novel, non-nucleoside polymerase basic protein 2 subunit inhibitor of the influenza A virus polymerase complex, and interaction with oseltamivir: a Phase 1 open-label study in healthy volunteers
Abstract
Aims: The aim of this study was to evaluate the drug-drug interaction between pimodivir, a novel, non-nucleoside polymerase basic protein 2 (PB2) subunit inhibitor of the influenza A virus polymerase complex, and oseltamivir, to assess the feasibility of this combination therapy. Furthermore, single- and multiple-dose pharmacokinetics and safety of pimodivir in healthy volunteers were assessed.
Methods: In Part 1 of this open-label Phase 1 study, healthy volunteers (n = 18) were randomized to one of six cross-over treatment sequences, each comprising administration of oseltamivir 75 mg or pimodivir 600 mg or combination thereof twice daily on Days 1-4, followed by a single morning dose on Day 5. Between each treatment session, there was a minimum 5-day washout period. In Part 2, healthy volunteers (n = 16) randomly received pimodivir 600 mg or placebo (3:1) twice daily on Days 1-9, followed by a single morning dose on Day 10. Pharmacokinetics of pimodivir, oseltamivir and oseltamivir carboxylate, and safety were assessed.
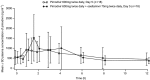
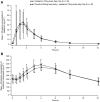
Results: In Part 1, co-administration of pimodivir with oseltamivir increased the Cmax of pimodivir by 31% (90% CI: 0.92-1.85) with no change in Cmin or AUC12h . Pimodivir had no effect on oseltamivir or oseltamivir carboxylate pharmacokinetics. In Part 2, after single- and multiple-dose administration of pimodivir, there was a 1.2- and 1.8-fold increase in Cmax and AUC12h , respectively, between Day 1 and Day 10. The most frequently reported treatment-emergent adverse event was diarrhoea (n = 7 each in Part 1 and 2).
Conclusion: Combination treatment with pimodivir and oseltamivir in healthy volunteers showed no clinically relevant drug-drug interactions. No safety concerns were identified with pimodivir 600 mg twice daily alone or in combination with oseltamivir 75 mg twice daily.
Keywords: combination; drug-drug interaction; oseltamivir; pharmacokinetics; pimodivir; safety.
© 2018 The British Pharmacological Society.
Figures


Similar articles
-
Phase 2b Study of Pimodivir (JNJ-63623872) as Monotherapy or in Combination With Oseltamivir for Treatment of Acute Uncomplicated Seasonal Influenza A: TOPAZ Trial.J Infect Dis. 2019 Mar 15;219(7):1026-1034. doi: 10.1093/infdis/jiy547. J Infect Dis. 2019. PMID: 30428049 Clinical Trial.
-
A Phase 2 Study of Pimodivir (JNJ-63623872) in Combination With Oseltamivir in Elderly and Nonelderly Adults Hospitalized With Influenza A Infection: OPAL Study.J Infect Dis. 2022 Aug 12;226(1):109-118. doi: 10.1093/infdis/jiaa376. J Infect Dis. 2022. PMID: 32604406 Free PMC article. Clinical Trial.
-
Genotypic and phenotypic characterization of influenza A viral variants in study participants treated with pimodivir in the phase 2b TOPAZ study.Antivir Ther. 2023 Jun;28(3):13596535231174273. doi: 10.1177/13596535231174273. Antivir Ther. 2023. PMID: 37226302 Clinical Trial.
-
Pimodivir treatment in adult volunteers experimentally inoculated with live influenza virus: a Phase IIa, randomized, double-blind, placebo-controlled study.Antivir Ther. 2018;23(4):335-344. doi: 10.3851/IMP3212. Antivir Ther. 2018. PMID: 29244026 Clinical Trial.
-
Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations.J Antimicrob Chemother. 2010 Apr;65 Suppl 2(Suppl 2):ii5-ii10. doi: 10.1093/jac/dkq015. J Antimicrob Chemother. 2010. PMID: 20215135 Free PMC article. Review.
Cited by
-
Preclinical and clinical developments for combination treatment of influenza.PLoS Pathog. 2022 May 12;18(5):e1010481. doi: 10.1371/journal.ppat.1010481. eCollection 2022 May. PLoS Pathog. 2022. PMID: 35551301 Free PMC article. Review.
-
The evolution, pathogenicity and transmissibility of quadruple reassortant H1N2 swine influenza virus in China: A potential threat to public health.Virol Sin. 2024 Apr;39(2):205-217. doi: 10.1016/j.virs.2024.02.002. Epub 2024 Feb 10. Virol Sin. 2024. PMID: 38346538 Free PMC article.
-
A phase I, single-center, randomized, open-label, three-period crossover study to evaluate the drug-drug interaction between ZSP1273 and oseltamivir in healthy Chinese subjects.Antimicrob Agents Chemother. 2025 Apr 2;69(4):e0172924. doi: 10.1128/aac.01729-24. Epub 2025 Feb 24. Antimicrob Agents Chemother. 2025. PMID: 39992105 Free PMC article. Clinical Trial.
-
Design, Synthesis, Biological Evaluation, and Molecular Dynamics Simulation of Influenza Polymerase PB2 Inhibitors.Molecules. 2023 Feb 15;28(4):1849. doi: 10.3390/molecules28041849. Molecules. 2023. PMID: 36838837 Free PMC article.
-
Novel Small Molecule Targeting the Hemagglutinin Stalk of Influenza Viruses.J Virol. 2019 Aug 13;93(17):e00878-19. doi: 10.1128/JVI.00878-19. Print 2019 Sep 1. J Virol. 2019. PMID: 31167918 Free PMC article.
References
-
- World Health Organization . Influenza infection in humans (February 2014) 2014. Available at http://www.who.int/influenza/human_animal_interface/virology_laboratorie... (last accessed 3 September 2018).
-
- Paules C, Subbarao K. Influenza. Lancet 2017; 390: 697–708. - PubMed
-
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289: 179–186. - PubMed
-
- Centers for Disasese Control and Prevention . 2009 H1N1‐related deaths, hospitalizations and cases: details of extrapolations and ranges: United States, Emerging Infections Program (EIP) data 2009. Available at https://www.cdc.gov/h1n1flu/pdf/details_eip_methods_plus_schematic.pdf (last accessed 3 September 2018).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

