An observational, multicentre study of cabazitaxel in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (CAPRISTANA)
- PMID: 30098093
- PMCID: PMC7379594
- DOI: 10.1111/bju.14509
An observational, multicentre study of cabazitaxel in patients with metastatic castration-resistant prostate cancer previously treated with docetaxel (CAPRISTANA)
Abstract
Objectives: To obtain routine clinical practice data on cabazitaxel usage patterns for patients with metastatic castration-resistant prostate cancer (mCRPC) and to describe physician-assessed cabazitaxel effectiveness, health-related quality of life (HRQoL) and safety.
Patients and methods: CAPRISTANA was an international, observational cohort study examining cabazitaxel use for the treatment of patients with mCRPC. Effectiveness was assessed by overall survival (OS), progression-free survival (PFS), time to treatment failure (TTF) and disease control rate. HRQoL was assessed using the Functional Assessment of Cancer Therapy-Prostate questionnaire (FACT-P) and the three-level European Quality of Life questionnaire (EQ-5D-3L). Safety was assessed by adverse event (AE) reporting.
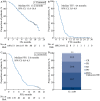
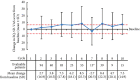
Results: A total of 189 patients were treated across 54 centres between April 2012 and June 2016. At baseline, 58.7% had ≥1 comorbidity, 93.7% had an Eastern Cooperative Oncology Group performance status ≤1, and 60.1% had a Gleason score at diagnosis of ≥8. Patients received a median of 6 cabazitaxel cycles; 84.7% received cabazitaxel as second-line therapy. The median OS, PFS and TTF were 13.2, 5.6 and 4.4 months, respectively. Cabazitaxel led to disease control in 52.9% of patients. HRQoL was maintained (40.3%) or improved (32.2%) in 72.5% of patients based on total FACT-P scores. Interestingly, 53.6% of patients reported pain improvement and a further 21.2% maintained pain control based on FACT-P prostate cancer-specific pain scores. The most common treatment-related grade ≥3 AEs were neutropenia (7.9%) and anaemia (2.1%).
Conclusion: Patients in CAPRISTANA treated with cabazitaxel had similar disease outcomes and safety profiles compared with large phase III clinical trials. Most patients had maintained or improved HRQoL scores; >70% of patients had maintained or improved pain control.
Keywords: mCRPC; #PCSM; #ProstateCancer; HRQoL; cabazitaxel; health-related quality of life; metastatic castration-resistant prostate cancer; real-world.
© 2018 Sanofi Genzyme BJU International published by John Wiley & Sons Ltd on behalf of BJU International.
Conflict of interest statement
Ayse Özatilgan and Simon Hitier are employed by Sanofi. Denise Bury and Gisoo Barnes are contracted by Sanofi. Marwan Ghosn has provided a consulting/advisory role for Sanofi, Astellas and Janssen. Joan Carles has provided a consulting/advisory role for Johnson&Johnson, Astellas, Bayer, Sanofi, Pfizer and BMS, and has delivered lectures for Bayer and Johnson&Johnson. Irina Koroleva has received personal fees from AstraZeneca, Teva, MSD and Eisai, and grants from AstraZeneca and Teva. Angelika Pichler, Antoaneta Tomova, Fadi El Karak, Hana Korunkova, Jana Katolicka and Joseph Makdessi have no conflict of interests to disclose.
Figures

References
-
- Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: E359–86 - PubMed
-
- Kirby M, Hirst C, Crawford ED. Characterising the castration‐resistant prostate cancer population: a systematic review. Int J Clin Pract 2011; 65: 1180–92 - PubMed
-
- National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Prostate Cancer (Version 2.2017), 2017.
-
- Scher HI, Fizazi K, Saad F et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012; 367: 1187–97 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

