Prostate cancer sheds the αvβ3 integrin in vivo through exosomes
- PMID: 30098419
- PMCID: PMC6541230
- DOI: 10.1016/j.matbio.2018.08.004
Prostate cancer sheds the αvβ3 integrin in vivo through exosomes
Abstract
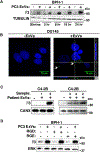
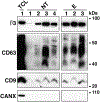
The αvβ3 integrin has been shown to promote aggressive phenotypes in many types of cancers, including prostate cancer. We show that GFP-labeled αvβ3 derived from cancer cells circulates in the blood and is detected in distant lesions in NOD scid gamma (NSG) mice. We, therefore, hypothesized that αvβ3 travels through exosomes and tested its levels in pools of vesicles, which we designate extracellular vesicles highly enriched in exosomes (ExVs), and in exosomes isolated from the plasma of prostate cancer patients. Here, we show that the αvβ3 integrin is found in patient blood exosomes purified by sucrose or iodixanol density gradients. In addition, we provide evidence that the αvβ3 integrin is transferred through ExVs isolated from prostate cancer patient plasma to β3-negative recipient cells. We also demonstrate the intracellular localization of β3-GFP transferred via cancer cell-derived ExVs. We show that the ExVs present in plasma from prostate cancer patients contain higher levels of αvβ3 and CD9 as compared to plasma ExVs from age-matched subjects who are not affected by cancer. Furthermore, using PSMA antibody-bead mediated immunocapture, we show that the αvβ3 integrin is expressed in a subset of exosomes characterized by PSMA, CD9, CD63, and an epithelial-specific marker, Trop-2. Finally, we present evidence that the levels of αvβ3, CD63, and CD9 remain unaltered in ExVs isolated from the blood of prostate cancer patients treated with enzalutamide. Our results suggest that detecting exosomal αvβ3 integrin in prostate cancer patients could be a clinically useful and non-invasive biomarker to follow prostate cancer progression. Moreover, the ability of αvβ3 integrin to be transferred from ExVs to recipient cells provides a strong rationale for further investigating the role of αvβ3 integrin in the pathogenesis of prostate cancer and as a potential therapeutic target.
Keywords: Abiraterone acetate; Enzalutamide; Extracellular vesicles; Plasma exosomes; Prostate cancer; αvβ3 integrin.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures





References
-
- Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018, CA Cancer J. Clin. 68 (1) (2018) 7–30. - PubMed
-
- Culig Z, Targeting the androgen receptor in prostate cancer, Expert. Opin. Pharmacother. 15 (10) (2014) 1427–1437. - PubMed
-
- Isaacs JT, The biology of hormone refractory prostate cancer. Why does it develop? Urol. Clin. North Am. 26 (2) (1999) 263–273. - PubMed
-
- Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J, Chowdhury S, Davis ID, de Bono JS, Evans CP, Fizazi K, Joshua AM, Kim CS, Kimura G, Mainwaring P, Mansbach H, Miller K, Noonberg SB, Perabo F, Phung D, Saad F, Scher HI, Taplin ME, Venner PM, Tombal B, Enzalutamide in metastatic prostate cancer before chemotherapy, N. Engl. J. Med. 371 (5) (2014) 424–433. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

