A three-dimensional (3D) organotypic microfluidic model for glioma stem cells - Vascular interactions
- PMID: 30098794
- PMCID: PMC6353712
- DOI: 10.1016/j.biomaterials.2018.07.048
A three-dimensional (3D) organotypic microfluidic model for glioma stem cells - Vascular interactions
Abstract
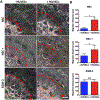
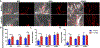
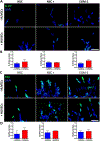
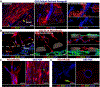
Glioblastoma (GBM) is one of the deadliest forms of cancer. Despite many treatment options, prognosis of GBM remains dismal with a 5-year survival rate of 4.7%. Even then, tumors often recur after treatment. Tumor recurrence is hypothesized to be driven by glioma stem cell (GSC) populations which are highly tumorigenic, invasive, and resistant to several forms of therapy. GSCs are often concentrated around the tumor vasculature, referred to as the vascular niche, which are known to provide microenvironmental cues to maintain GSC stemness, promote invasion, and resistance to therapies. In this work, we developed a 3D organotypic microfluidic platform, integrated with hydrogel-based biomaterials, to mimic the GSC vascular niche and study the influence of endothelial cells (ECs) on patient-derived GSC behavior and identify signaling cues that mediate their invasion and phenotype. The established microvascular network enhanced GSC migration within a 3D hydrogel, promoted invasive morphology as well as maintained GSC proliferation rates and phenotype (Nestin, SOX2, CD44). Notably, we compared migration behavior to in vivo mice model and found similar invasive morphology suggesting that our microfluidic system could represent a physiologically relevant in vivo microenvironment. Moreover, we confirmed that CXCL12-CXCR4 signaling is involved in promoting GSC invasion in a 3D vascular microenvironment by utilizing a CXCR4 antagonist (AMD3100), while also demonstrating the effectiveness of the microfluidic as a drug screening assay. Our model presents a potential ex vivo platform for studying the interplay of GSCs with its surrounding microenvironment as well as development of future therapeutic strategies tailored toward disrupting key molecular pathways involved in GSC regulatory mechanisms.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures




References
-
- Omuro A, DeAngelis LM, Glioblastoma and other malignant gliomas: a clinical review, JAMA 310(17) (2013) 1842–50. - PubMed
-
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN, Glioma stem cells promote radioresistance by preferential activation of the DNA damage response, Nature 444(7120) (2006) 756–60. - PubMed
-
- Sundar SJ, Hsieh JK, Manjila S, Lathia JD, Sloan A, The role of cancer stem cells in glioblastoma, Neurosurg Focus 37(6) (2014) E6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

