Decarbamoylation of acetylcholinesterases is markedly slowed as carbamoyl groups increase in size
- PMID: 30098983
- PMCID: PMC6204216
- DOI: 10.1016/j.abb.2018.08.006
Decarbamoylation of acetylcholinesterases is markedly slowed as carbamoyl groups increase in size
Abstract
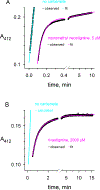
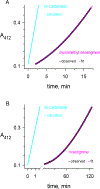
Carbamates are esters of substituted carbamic acids that react with acetylcholinesterase (AChE) by initially transferring the carbamoyl group to a serine residue in the enzyme active site accompanied by loss of the carbamate leaving group followed by hydrolysis of the carbamoyl enzyme. This hydrolysis, or decarbamoylation, is relatively slow, and half-lives of carbamoylated AChEs range from 4 min to more than 30 days. Therefore, carbamates are effective AChE inhibitors that have been developed as insecticides and as therapeutic agents. We show here, in contrast to a previous report, that decarbamoylation rate constants are independent of the leaving group for a series of carbamates with the same carbamoyl group. When the alkyl substituents on the carbamoyl group increased in size from N-monomethyl- to N,N-dimethyl-, N-ethyl-N-methyl-, or N,N-diethyl-, the decarbamoylation rate constants decreased by 4-, 70-, and 800-fold, respectively. We suggest that this relationship arises as a result of active site distortion, particularly in the acyl pocket of the active site. Furthermore, solvent deuterium oxide isotope effects for decarbamoylation decreased from 2.8 for N-monomethylcarbamoyl AChE to 1.1 for N,N-diethylcarbamoyl AChE, indicating a shift in the rate-limiting step from general acid-base catalysis to a likely conformational change in the distorted active site.
Keywords: Acetylcholinesterase; Alzheimer's disease; Decarbamoylation.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests
Figures
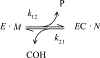
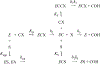
Similar articles
-
Rate-limiting step in the decarbamoylation of acetylcholinesterases with large carbamoyl groups.Chem Biol Interact. 2019 Aug 1;308:392-395. doi: 10.1016/j.cbi.2019.06.004. Epub 2019 Jun 6. Chem Biol Interact. 2019. PMID: 31175846 Free PMC article. Review.
-
Solvent Deuterium Oxide Isotope Effects on the Reactions of Organophosphorylated Acetylcholinesterase.Molecules. 2020 Sep 25;25(19):4412. doi: 10.3390/molecules25194412. Molecules. 2020. PMID: 32992925 Free PMC article.
-
Multiple binding sites involved in the effect of choline esters on decarbamoylation of monomethylcarbamoyl- or dimethylcarbamoly-acetylcholinesterase.Biochem J. 1994 Aug 1;301 ( Pt 3)(Pt 3):713-20. doi: 10.1042/bj3010713. Biochem J. 1994. PMID: 8053896 Free PMC article.
-
Interactions between the peripheral site and the acylation site in acetylcholinesterase.Chem Biol Interact. 2005 Dec 15;157-158:181-9. doi: 10.1016/j.cbi.2005.10.027. Epub 2005 Oct 27. Chem Biol Interact. 2005. PMID: 16256966
-
Amino acid residues involved in the interaction of acetylcholinesterase and butyrylcholinesterase with the carbamates Ro 02-0683 and bambuterol, and with terbutaline.Biochim Biophys Acta. 1999 Aug 17;1433(1-2):261-71. doi: 10.1016/s0167-4838(99)00124-7. Biochim Biophys Acta. 1999. PMID: 10446376
Cited by
-
Rivastigmine-Bambuterol Hybrids as Selective Butyrylcholinesterase Inhibitors.Molecules. 2023 Dec 22;29(1):72. doi: 10.3390/molecules29010072. Molecules. 2023. PMID: 38202655 Free PMC article.
-
Treatment of acute organophosphate poisoning by using a cocaine hydrolase engineered from human butyrylcholinesterase.Chem Biol Interact. 2025 Aug 1;416:111552. doi: 10.1016/j.cbi.2025.111552. Epub 2025 May 8. Chem Biol Interact. 2025. PMID: 40339683
-
Rate-limiting step in the decarbamoylation of acetylcholinesterases with large carbamoyl groups.Chem Biol Interact. 2019 Aug 1;308:392-395. doi: 10.1016/j.cbi.2019.06.004. Epub 2019 Jun 6. Chem Biol Interact. 2019. PMID: 31175846 Free PMC article. Review.
-
Profiling the Tox21 Chemical Collection for Acetylcholinesterase Inhibition.Environ Health Perspect. 2021 Apr;129(4):47008. doi: 10.1289/EHP6993. Epub 2021 Apr 12. Environ Health Perspect. 2021. PMID: 33844597 Free PMC article.
-
Solvent Deuterium Oxide Isotope Effects on the Reactions of Organophosphorylated Acetylcholinesterase.Molecules. 2020 Sep 25;25(19):4412. doi: 10.3390/molecules25194412. Molecules. 2020. PMID: 32992925 Free PMC article.
References
-
- Rosenberry TL, and Scoggin DM (1984) Structure of human erythrocyte acetylcholinesterase. Characterization of intersubunit disulfide bonding and detergent interaction, J. Biol. Chem 259, 5643–5652. - PubMed
-
- De Ferrari GV, Mallender WD, Inestrosa NC, and Rosenberry TL (2001) Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites, J. Biol. Chem 276, 23282–23287. - PubMed
-
- Taylor P, and Lappi S (1975) Interaction of fluorescence probes with acetylcholinesterase.The site and specificity of propidium binding, Biochemistry 14, 1989–1997. - PubMed
-
- Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, and Silman I (1991) Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein, Science 253, 872–879. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

