Characterizing the course of suicidal ideation response to ketamine
- PMID: 30099268
- PMCID: PMC6193483
- DOI: 10.1016/j.jad.2018.07.077
Characterizing the course of suicidal ideation response to ketamine
Abstract
Background: No pharmacological treatments exist for active suicidal ideation (SI), but the glutamatergic modulator ketamine elicits rapid changes in SI. We developed data-driven subgroups of SI trajectories after ketamine administration, then evaluated clinical, demographic, and neurobiological factors that might predict SI response to ketamine.
Methods: Data were pooled from five clinical ketamine trials. Treatment-resistant inpatients (n = 128) with DSM-IV-TR-diagnosed major depressive disorder (MDD) or bipolar depression received one subanesthetic (0.5 mg/kg) ketamine infusion over 40 min. Composite SI variable scores were analyzed using growth mixture modeling to generate SI response classes, and class membership predictors were evaluated using multinomial logistic regressions. Putative predictors included demographic variables and various peripheral plasma markers.
Results: The best-fitting growth mixture model comprised three classes: Non-Responders (29%), Responders (44%), and Remitters (27%). For Responders and Remitters, maximal improvements were achieved by Day 1. Improvements in SI occurred independently of improvements in a composite Depressed Mood variable for Responders, and partially independently for Remitters. Indicators of chronic SI and self-injury were associated with belonging to the Non-Responder group. Higher levels of baseline plasma interleukin-5 (IL-5) were linked to Remitters rather than Responders.
Limitations: Subjects were not selected for active suicidal thoughts; findings only extend to Day 3; and plasma, rather than CSF, markers were used.
Conclusion: The results underscore the heterogeneity of SI response to ketamine and its potential independence from changes in Depressed Mood. Individuals reporting symptoms suggesting a longstanding history of chronic SI were less likely to respond or remit post-ketamine.
Trial registration: ClinicalTrials.gov NCT00088699.
Keywords: Depression; Growth mixture modeling; Ketamine; Suicidal ideation; Suicide.
Published by Elsevier B.V.
Conflict of interest statement
Declaration of interest
Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine in major depression and suicidal ideation; as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (
All other authors have no conflict of interest to disclose, financial or otherwise.
Figures
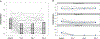
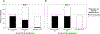
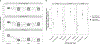
References
-
- IBM SPSS Statistics for Windows, 2016. Version 21.0. IBM Corporation, Armonk NY Ballard ED, Yarrington JS, Farmer CA, Lener MS, Kadriu B, Lally N, Williams D, Machado-Vieira R, Niciu MJ, Park L, Zarate CA Jr., 2018. Parsing the heterogeneity of depression: an exploratory factor analysis across commonly used depression rating scales. J. Affect. Disord 231, 51–57. - PMC - PubMed
-
- Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Traskman-Bendz L, Guillemin GJ, Erhardt S, Brundin L, 2015. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav. Immun 43, 110–117. - PubMed
-
- Beck AT, Kovacs M, Weissman A, 1979. Assessment of suicidal intention: the scale for suicide ideation. J. Consult. Clin. Psychol 47, 343–352. - PubMed
-
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J, 1994. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry 151, 1132–1136. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

