Manual ventilation to prevent hypoxaemia during endotracheal intubation of critically ill adults: protocol and statistical analysis plan for a multicentre randomised trial
- PMID: 30099400
- PMCID: PMC6089322
- DOI: 10.1136/bmjopen-2018-022139
Manual ventilation to prevent hypoxaemia during endotracheal intubation of critically ill adults: protocol and statistical analysis plan for a multicentre randomised trial
Abstract
Introduction: Hypoxaemia is the most common complication during endotracheal intubation of critically ill adults, and it increases the risk of cardiac arrest and death. Manual ventilation between induction and intubation has been hypothesised to decrease the incidence of hypoxaemia, but efficacy and safety data are lacking.
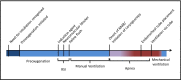
Methods and analysis: The Preventing Hypoxemia with Manual Ventilation during Endotracheal Intubation trial is a prospective, multicentre, non-blinded randomised clinical trial being conducted in seven intensive care units in the USA. A total of 400 critically ill adults undergoing endotracheal intubation will be randomised 1:1 to receive prophylactic manual ventilation between induction and endotracheal intubation using a bag-valve-mask device or no prophylactic ventilation. The primary outcome is the lowest arterial oxygen saturation between induction and 2 min after successful endotracheal intubation, which will be analysed as an unadjusted, intention-to-treat comparison of patients randomised to prophylactic ventilation versus patients randomised to no prophylactic ventilation. The secondary outcome is the incidence of severe hypoxaemia, defined as any arterial oxygen saturation of less than 80% between induction and 2 min after endotracheal intubation. Enrolment began on 2 February 2017 and is expected to be complete in May 2018.
Ethics and dissemination: The trial was approved by the institutional review boards or designees of all participating centres. The results will be submitted for publication in a peer-reviewed journal and presented at one or more scientific conferences.
Trial registration number: NCT03026322; Pre-results.
Keywords: adult anaesthesia; adult thoracic medicine.
© Author(s) (or their employer(s)) 2018. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors declared no potential conflicts of interest with the current work. TWR reported serving on an advisory board for Avisa Pharma, LLC, and as the Director of Medical Affairs for Cumberland Pharmaceuticals.
Figures


References
-
- Schwartz DE, Matthay MA, Cohen NH. Death and other complications of emergency airway management in critically ill adults. A prospective investigation of 297 tracheal intubations. Anesthesiology 1995;82:367–76. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
