A cellular mechanism of muscle memory facilitates mitochondrial remodelling following resistance training
- PMID: 30099751
- PMCID: PMC6138296
- DOI: 10.1113/JP275308
A cellular mechanism of muscle memory facilitates mitochondrial remodelling following resistance training
Abstract
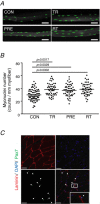
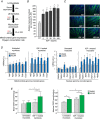
Key points: Referring to the muscle memory theory, previously trained muscles acquire strength and volume much faster than naive muscles. Using extreme experimental models such as synergist ablation or steroid administration, previous studies have demonstrated that the number of nuclei increases when a muscle becomes enlarged, which serves as a cellular muscle memory mechanism for the muscle. In the present study, we found that, when rats were subjected to physiologically relevant resistance training, the number of myonuclei increased and was retained during a long-term detraining period. The acquired myonuclei were related to a greater degree of muscle hypertrophic and mitochondrial biogenesis processes following subsequent hypertrophic conditions. Our data suggest a cellular mechanism supporting the notion that exposing young muscles to resistance training would help to restore age-related muscle loss coupled with mitochondrial dysfunction in later life.
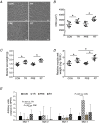
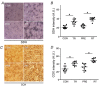
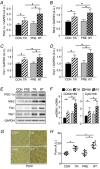
Abstract: Muscle hypertrophy induced by resistance training is accompanied by an increase in the number of myonuclei. The acquired myonuclei are viewed as a cellular component of muscle memory by which muscle enlargement is promoted during a re-training period. In the present study, we investigated the effect of exercise preconditioning on mitochondrial remodelling induced by resistance training. Sprague-Dawley rats were divided into four groups: untrained control, training, pre-training or re-training. The training groups were subjected to weight loaded-ladder climbing exercise training. Myonuclear numbers were significantly greater (up to 20%) in all trained muscles compared to untrained controls. Muscle mass was significantly higher in the re-training group compared to the training group (∼2-fold increase). Mitochondrial content, mitochondrial biogenesis gene expression levels and mitochondrial DNA copy numbers were significantly higher in re-trained muscles compared to the others. Oxidative myofibres (type I) were significantly increased only in the re-trained muscles. Furthermore, in vitro studies using insulin-like growth factor-1-treated L6 rat myotubes demonstrated that myotubes with a higher myonuclear number confer greater expression levels of both mitochondrial and nuclear genes encoding for constitutive and regulatory mitochondrial proteins, which also showed a greater mitochondrial respiratory function. These data suggest that myonuclei acquired from previous training facilitate mitochondrial biogenesis in response to subsequent retraining by (at least in part) enhancing cross-talk between mitochondria and myonuclei in the pre-conditioned myofibres.
Keywords: mitochondrial biogenesis; muscle memory; myonuclei; resistance training.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures


Comment in
-
Muscle memory: virtues of your youth?J Physiol. 2018 Sep;596(18):4289-4290. doi: 10.1113/JP276354. Epub 2018 Aug 25. J Physiol. 2018. PMID: 30145845 Free PMC article. No abstract available.
References
-
- Allen DL, Monke SR, Talmadge RJ, Roy RR & Edgerton VR. (1995). Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal muscle fibers. J Appl Physiol 78, 1969–1976. - PubMed
-
- Amthor H, Otto A, Vulin A, Rochat A, Dumonceaux J, Garcia L, Mouisel E, Hourde C, Macharia R, Friedrichs M, Relaix F, Zammit PS, Matsakas A, Patel K & Partridge T. (2009). Muscle hypertrophy driven by myostatin blockade does not require stem/precursor‐cell activity. Proc Natl Acad Sci U S A 106, 7479–7484. - PMC - PubMed
-
- Barres R, Osler ME, Yan J, Rune A, Fritz T, Caidahl K, Krook A & Zierath JR. (2009). Non‐CpG methylation of the PGC‐1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab 10, 189–198. - PubMed
-
- Blaauw B, Canato M, Agatea L, Toniolo L, Mammucari C, Masiero E, Abraham R, Sandri M, Schiaffino S & Reggiani C. (2009). Inducible activation of Akt increases skeletal muscle mass and force without satellite cell activation. FASEB J 23, 3896–3905. - PubMed
-
- Bolster DR, Jefferson LS & Kimball SR. (2004). Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin‐, amino acid‐ and exercise‐induced signalling. Proc Nutr Soc 63, 351–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

