Prophylactic and therapeutic effects of twice-daily famciclovir administration on infectious upper respiratory disease in shelter-housed cats
- PMID: 30099962
- PMCID: PMC10814541
- DOI: 10.1177/1098612X18789719
Prophylactic and therapeutic effects of twice-daily famciclovir administration on infectious upper respiratory disease in shelter-housed cats
Erratum in
-
Prophylactic and therapeutic effects of twice-daily famciclovir administration on infectious upper respiratory disease in shelter-housed cats.J Feline Med Surg. 2019 Jun;21(6):NP2. doi: 10.1177/1098612X19832156. Epub 2019 Feb 14. J Feline Med Surg. 2019. PMID: 30762464 Free PMC article. No abstract available.
Abstract
Objectives: In humans with herpetic disease, early or pre-emptive famciclovir therapy reduces disease duration and severity. This prospective, masked, placebo-controlled study tested therapeutic and prophylactic effects of two famciclovir doses given to cats for 7 days following shelter entry.
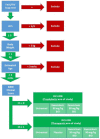
Methods: Cats were assigned to prophylactic or therapeutic study arms based on clinical evidence of herpetic disease at study entry. Cats in the therapeutic arm received no treatment (n = 19), placebo (lactose; n = 18) or famciclovir at ~30 (n = 21) or ~90 mg/kg (n = 20) PO q12h for 7 days. Cats in the prophylactic arm received no treatment (n = 25) or famciclovir at ~30 (n = 28) or ~90 mg/kg (n = 27) PO q12h for 7 days. Disease scores, body weight, conjunctival feline herpesvirus 1 (FHV-1) shedding, and adoption rates were recorded on days 1 (admission), 8 (end of therapy) and 15 (1 week after cessation of therapy).
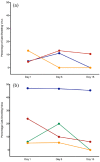
Results: No significant differences in clinical scores were observed among groups in the prophylactic or therapeutic arms at any of the three time points. However, within the therapeutic arm, viral shedding on day 8 was significantly higher in cats receiving no treatment than in those receiving ~30 or ~90 mg/kg famciclovir, and this effect persisted 1 week after famciclovir was stopped (day 15) only in cats receiving ~30 mg/kg, although this approached significance in cats receiving ~90 mg/kg. No significant differences in adoption rates were detected among groups in either arm throughout the study.
Conclusions and relevance: Although we did not demonstrate a statistically or clinically significant effect of famciclovir administration upon clinical signs of infectious upper respiratory disease or adoption, when it was administered at ~30 or ~90 mg/kg q12h for 1 week famciclovir reduced conjunctival FHV-1 shedding. This suggests a potential role in interrupting the infectious cycle within a shelter population; however, cost in time and resources, and stress and pathogen transmission induced by oral administration should be considered.
Keywords: Antiviral therapy; feline herpesvirus; ocular disease; penciclovir; population health; viral prophylaxis.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Gaskell RM, Wardley RC. Feline viral respiratory disease: a review with particular reference to its epizootiology and control. J Small Anim Pract 1978; 19: 1–16. - PubMed
-
- Gaskell RM, Povey RC. Experimental induction of feline viral rhinotracheitis virus re-excretion in FVR-recovered cats. Vet Rec 1977; 100: 128–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

