Amino Acid Metabolism as a Target for Breast Cancer Imaging
- PMID: 30100081
- PMCID: PMC6092023
- DOI: 10.1016/j.cpet.2018.02.009
Amino Acid Metabolism as a Target for Breast Cancer Imaging
Abstract
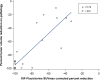
Amino acids are an alternate energy source to glucose, and amino acid metabolism is up-regulated in multiple malignancies, including breast cancers. Multiple amino acid radiotracers have been used to image breast cancer with unique strengths and weaknesses. 11C-methionine uptake correlates with S-phase fraction in breast cancer and may be useful for evaluation of treatment response. Invasive lobular breast cancers may demonstrate greater 18F-fluciclovine avidity than 18F-fluorodeoxyglucose. Thus, different histologic subtypes of breast cancer may use diverse metabolic pathways and may be better imaged by different tracers.
Keywords: Amino acid; Breast cancer; Fluciclovine; Methionine; PET/CT.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures


References
-
- Gambhir SS, Czernin J, Schwimmer J, Silverman DH, Coleman RE, Phelps ME. A tabulated summary of the FDG PET literature. J Nucl Med. 2001;42(5 Suppl):1S–93S. - PubMed
-
- Kang BJ, Lee JH, Yoo Ie R, et al. Clinical significance of incidental finding of focal activity in the breast at 18F-FDG PET/CT. AJR Am J Roentgenol. 2011;197(2):341–347. - PubMed
-
- Fletcher JW, Djulbegovic B, Soares HP, et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49(3):480–508. - PubMed
-
- Wahl RL, Siegel BA, Coleman RE, Gatsonis CG. Prospective multicenter study of axillary nodal staging by positron emission tomography in breast cancer: a report of the staging breast cancer with PET Study Group. J Clin Oncol. 2004;22(2):277–285. - PubMed
-
- Peare R, Staff RT, Heys SD. The use of FDG-PET in assessing axillary lymph node status in breast cancer: a systematic review and meta-analysis of the literature. Breast Cancer Res Treat. 2010;123(1):281–290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

