Phase-Locked Stimulation during Cortical Beta Oscillations Produces Bidirectional Synaptic Plasticity in Awake Monkeys
- PMID: 30100342
- PMCID: PMC6108550
- DOI: 10.1016/j.cub.2018.07.009
Phase-Locked Stimulation during Cortical Beta Oscillations Produces Bidirectional Synaptic Plasticity in Awake Monkeys
Abstract
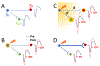
The functional role of cortical beta oscillations, if any, remains unresolved. During oscillations, the periodic fluctuation in excitability of entrained cells modulates transmission of neural impulses and periodically enhances synaptic interactions. The extent to which oscillatory episodes affect activity-dependent synaptic plasticity remains to be determined. In nonhuman primates, we delivered single-pulse electrical cortical stimulation to a "stimulated" site in sensorimotor cortex triggered on a specific phase of ongoing beta (12-25 Hz) field potential oscillations recorded at a separate "triggering" site. Corticocortical connectivity from the stimulated to the triggering site as well as to other (non-triggering) sites was assessed by cortically evoked potentials elicited by test stimuli to the stimulated site, delivered outside of oscillatory episodes. In separate experiments, connectivity was assessed by intracellular recordings of evoked excitatory postsynaptic potentials. The conditioning paradigm produced transient (1-2 s long) changes in connectivity between the stimulated and the triggering site that outlasted the duration of the oscillatory episodes. The direction of the plasticity effect depended on the phase from which stimulation was triggered: potentiation in depolarizing phases, depression in hyperpolarizing phases. Plasticity effects were also seen at non-triggering sites that exhibited oscillations synchronized with those at the triggering site. These findings indicate that cortical beta oscillations provide a spatial and temporal substrate for short-term, activity-dependent synaptic plasticity in primate neocortex and may help explain the role of oscillations in attention, learning, and cortical reorganization.
Keywords: beta oscillations; closed loop; cortical connectivity; cortical stimulation; nonhuman primates; synaptic plasticity.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures




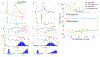
Comment in
-
Latent Connectivity: Neuronal Oscillations Can Be Leveraged for Transient Plasticity.Curr Biol. 2018 Aug 20;28(16):R879-R882. doi: 10.1016/j.cub.2018.06.073. Curr Biol. 2018. PMID: 30130509
References
-
- Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, and Singer W (2010). Neural synchrony and the development of cortical networks. Trends in cognitive sciences 14, 72–80. - PubMed
-
- Sanes JN, and Donoghue JP (2000). Plasticity and primary motor cortex. Annual review of neuroscience 23, 393–415. - PubMed
-
- Nudo R (2014). Plasticity of cerebral motor functions: implications for repair and rehabilitation. In Textbook of Neural Repair and Rehabilitation, 2e, Volume 1, Selzer ME, Clarke S, Cohen LG, Kwakkel G and Miller RH, eds. (Cambridge, UK: Cambridge University Press; ), pp. 99–113.
-
- Donoghue JP, Sanes JN, Hatsopoulos NG, and Gaal G (1998). Neural discharge and local field potential oscillations in primate motor cortex during voluntary movements. Journal of neurophysiology 79, 159–173. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

