Efficacy of bevacizumab versus epidermal growth factor receptor inhibitors for wild-type RAS metastatic colorectal cancer: a meta-analysis
- PMID: 30100734
- PMCID: PMC6065471
- DOI: 10.2147/OTT.S168695
Efficacy of bevacizumab versus epidermal growth factor receptor inhibitors for wild-type RAS metastatic colorectal cancer: a meta-analysis
Abstract
Background: Results from several prospective clinical trials comparing anti-epidermal growth factor receptor (EGFR) therapy and anti-vascular endothelial growth factor (VEGF) therapy plus chemotherapy for wild-type RAS metastatic colorectal cancer (mCRC) have been inconsistent. This meta-analysis aims to investigate the optimal choice for these target agents.
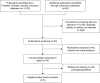
Methods: We searched for clinical trials in both electronic databases from inception until January 2018 and recent conference abstracts to identify prospective clinical studies comparing the efficacy of a VEGF inhibitor (bevacizumab) versus EGFR inhibitors (cetuximab or panitumumab) on wild-type RAS (including its subset KRAS) mCRC. All analyses were conducted using RevMan 5.3 software.
Results: A total of 5 studies were included. EGFR inhibitors were associated with a significant benefit in terms of overall survival (OS) compared with VEGF inhibitors in wild-type KRAS or wild-type RAS populations, with hazard ratios (HRs) equal to 0.86 (95% CI: 0.78, 0.95; p=0.003) and 0.83 (95% CI: 0.72, 0.95; p=0.007), respectively. This survival benefit was limited to the first-line setting. No difference was found for progression-free survival (PFS), whereas the objective response rate (ORR) was significantly increased in the wild-type RAS population (OR: 0.64; 95% CI: 0.50, 0.82; p=0.0004). No difference in OS was noted between EGFR inhibitors versus a VEGF inhibitor plus the FOLFIRI regimen, whereas superior survival was noted for EGFR inhibitors plus the mFOLFOX6 regimen versus a VEGF inhibitor (HR: 0.75; 95% CI: 0.57, 0.98; p=0.04). PFS was significantly prolonged (HR: 1.48; 95% CI: 1.14, 1.92; p=0.003), whereas a trend favoring OS (HR: 1.23; 95% CI: 0.93, 1.63; p=0.14) was noted for a VEGF inhibitor in patients with right-sided tumors, with no difference in the ORR (OR: 0.85; 95% CI: 0.52, 1.38; p=0.51). However, left-sided tumors exhibited superior OS (HR: 0.71; 95% CI: 0.59, 0.85; p=0.0002), PFS (HR: 0.84; 95% CI: 0.72, 0.98; p=0.03), and ORR (OR: 0.66; 95% CI: 0.48, 0.92; p=0.01) for EGFR inhibitors.
Conclusion: This meta-analysis suggests the superiority of anti-EGFR therapy compared with anti-VEGF therapy for mCRC with wild-type RAS. Primary tumor location should be taken into account in target drug selection. Further research is still needed to confirm which inhibitor may be a better choice when combined with different chemotherapy regimens.
Keywords: bevacizumab; cetuximab; colorectal cancer; meta-analysis; panitumumab.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures





References
-
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343(13):905–914. - PubMed
-
- de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18(16):2938–2947. - PubMed
-
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. - PubMed
-
- Van Cutsem E, Kohne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408–1417. - PubMed
-
- Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28(31):4706–4713. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

