Ex vivo decontamination of yeast-colonized dentures by iodine-thiocyanate complexes
- PMID: 30100762
- PMCID: PMC6064153
- DOI: 10.2147/CCIDE.S165377
Ex vivo decontamination of yeast-colonized dentures by iodine-thiocyanate complexes
Abstract
Introduction: Under well-defined experimental conditions, and in the presence of hydrogen peroxide, lactoperoxidase produces stable iodine-thiocyanate complexes that have antimicrobial properties. A novel process was developed to short circuit the consumption of hydrogen peroxide by microbial catalases by producing iodine-thiocyanate complexes prior to contact with microorganisms, with the aim of being able to decontaminate the ex vivo dentures colonized by yeasts.
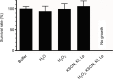
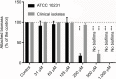
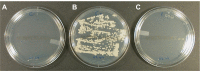
Materials and methods: Teabags containing lactoperoxidase adsorbed on inert clay beads were immersed for 1 minute in phosphate buffer solution (0.1 M pH 7.4) containing 5.2 mM potassium iodide, 1.2 mM potassium thiocyanate, and 5.5 mM hydrogen peroxide. After removing the adsorbed lactoperoxidase, the stability and efficacy of iodine-thiocyanate complexes for Candida-colonized denture decontamination were verified. Investigations were performed in vitro on Candida albicans ATCC 10231 and on clinical isolates from 46 dentures. A Candida plate count was performed after a 24-hour incubation at 37°C on Sabouraud-chloramphenicol or CHROMagar solid media; then, the yeast growth was evaluated in Sabouraud broth by turbidimetry and biofilm biomass by crystal violet staining.
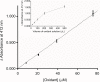
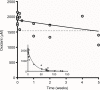
Results: In vitro tests demonstrated the effectiveness of the oxidant solution in sterilizing a suspension of 106Candida cells per milliliter after a 5-minute incubation. A single ex vivo immersion of contaminated dentures in a solution of iodine-thiocyanate complexes led to a decrease of at least 1 log unit in the number of colony-forming units in 58.3% of the tested dentures, while immersing in water alone had no effect on denture colonization (significant c2: p = 0.0006).
Conclusion: These data suggest a promising new strategy for decontamination of dentures.
Keywords: Candida; biofilm; hygiene; lactoperoxidase; oral cavity.
Conflict of interest statement
Disclosure Françoise Bafort and Jean-Paul Perraudin are two of the patented owners of the biological applications of iodine–thiocyanate complexes (Bafort F, Perraudin J-P, Jijakli H Composition comprenant des ions I2SCN− et/ou des ions I(SCN)−2. WO2017046407A1, 2015). The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Candida albicans susceptibility to lactoperoxidase-generated hypoiodite.Clin Cosmet Investig Dent. 2010 Aug 4;2:69-78. doi: 10.2147/cciden.s10891. Print 2010. Clin Cosmet Investig Dent. 2010. PMID: 23662084 Free PMC article.
-
Effect of lactoperoxidase on the antimicrobial effectiveness of the thiocyanate hydrogen peroxide combination in a quantitative suspension test.BMC Microbiol. 2009 Jul 9;9:134. doi: 10.1186/1471-2180-9-134. BMC Microbiol. 2009. PMID: 19589149 Free PMC article.
-
Microbicidal efficacy of thiocyanate hydrogen peroxide after adding lactoperoxidase under saliva loading in the quantitative suspension test.Arch Oral Biol. 2011 Dec;56(12):1576-82. doi: 10.1016/j.archoralbio.2011.04.016. Epub 2011 May 28. Arch Oral Biol. 2011. PMID: 21621190
-
[Oral candidiasis and dentures].Rev Stomatol Chir Maxillofac. 2010 Apr;111(2):74-8. doi: 10.1016/j.stomax.2009.10.007. Epub 2010 Mar 27. Rev Stomatol Chir Maxillofac. 2010. PMID: 20347465 Review. French.
-
Chemical cleaning methods for prostheses colonized by Candida spp.: A systematic review.J Prosthet Dent. 2020 Dec;124(6):653-658. doi: 10.1016/j.prosdent.2019.10.004. Epub 2020 Jan 25. J Prosthet Dent. 2020. PMID: 31987583
Cited by
-
Oral peroxidases: From antimicrobial agents to ecological actors (Review).Mol Med Rep. 2021 Jul;24(1):500. doi: 10.3892/mmr.2021.12139. Epub 2021 May 13. Mol Med Rep. 2021. PMID: 33982776 Free PMC article. Review.
References
-
- Smith QT, Yang CH. Salivary myeloperoxidase of young adult humans. Proc Soc Exp Biol Med. 1984;175(4):468–475. - PubMed
-
- Thomas EL, Jefferson MM, Joyner RE, Cook GS, King CC. Leukocyte myeloperoxidase and salivary lactoperoxidase: identification and quantification in human mixed saliva. J Dent Res. 1994;73(2):544–555. - PubMed
-
- Ashby MT. Inorganic chemistry of defensive peroxidases in the human oral cavity. J Dent Res. 2008;87(10):900–914. - PubMed
-
- Pruitt KM, Reiter B. Biochemistry of peroxidase system: antimicrobial effects. In: Pruitt KM, Tenovuo JO, editors. The Lactoperoxidase System: Chemistry and Biological Significance. New York: Marcel Dekker; 1985. pp. 143–178.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

