Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration
- PMID: 30100812
- PMCID: PMC6082025
- DOI: 10.1016/j.mattod.2017.10.005
Engineering vascularized and innervated bone biomaterials for improved skeletal tissue regeneration
Abstract
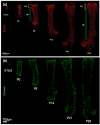
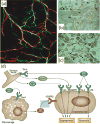
Blood vessels and nerve fibers are distributed throughout the entirety of skeletal tissue, and play important roles during bone development and fracture healing by supplying oxygen, nutrients, and cells. However, despite the successful development of bone mimetic materials that can replace damaged bone from a structural point of view, most of the available bone biomaterials often do not induce sufficient formation of blood vessels and nerves. In part, this is due to the difficulty of integrating and regulating multiple tissue types within artificial materials, which causes a gap between native skeletal tissue. Therefore, understanding the anatomy and underlying interaction mechanisms of blood vessels and nerve fibers in skeletal tissue is important to develop biomaterials that can recapitulate its complex microenvironment. In this perspective, we highlight the structure and osteogenic functions of the vascular and nervous system in bone, in a coupled manner. In addition, we discuss important design criteria for engineering vascularized, innervated, and neurovascularized bone implant materials, as well as recent advances in the development of such biomaterials. We expect that bone implant materials with neurovascularized networks can more accurately mimic native skeletal tissue and improve the regeneration of bone tissue.
Keywords: Bone biomaterials; innervated bone materials; neurovascularized bone materials; vascularized bone materials.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
