Interaction of Mannitol and Sucrose with Gellan Gum in Freeze-Dried Gel Systems
- PMID: 30100823
- PMCID: PMC6061513
- DOI: 10.1007/s11483-018-9536-5
Interaction of Mannitol and Sucrose with Gellan Gum in Freeze-Dried Gel Systems
Abstract
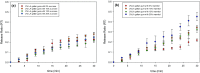
The effect of sucrose and mannitol addition to low-acyl (LA) gellan gum gels at both the molecular and macroscopic levels prior to, and after freeze-drying has been investigated. It has been shown that the gel network order as well as the mechanical properties are changed with the solute content, especially in the case of sucrose. The freeze-dried gel structure, containing either mannitol or sucrose, was studied, reporting for the first time the interaction of mannitol with the gellan gum gel. The generated freeze-dried gel network was evaluated in terms of porosity, pore size and wall thickness distributions. The solute physical state was correlated the water activity trend as a function of the solute content. Since mannitol is crystalline, the water activity decreases, in contrast with the amorphous sucrose. The rehydration mechanism was investigated and associated with the solute release from the structure. Specifically, the material properties (surface and bulk) as well as the role of the dissolution medium over time were assessed. It was found that the rehydration for both the gellan/sucrose and gellan/mannitol systems was highly influenced by the additive content, as an increase in water uptake was measured up to 10 wt%. A further increase in solute led to a considerable drop in the rehydration rate and extent due to the change in the freeze-dried structure, with smaller pores and with higher wall thickness values.
Keywords: Freeze drying; Gellan gum; Mannitol; Microstructure; Sucrose.
Figures










References
-
- Phillips GO, Williams PA. Woodhead Publishing Limited. 2000. Handbook of hydrocolloids.
-
- Norton I, Foster T. Spec. Publ. R. Soc. Chem. 2002;278:187–200.
-
- Kaushik V, Roos YH. LWT-Food Sci. Technol. 2007;40(8):1381–1391. doi: 10.1016/j.lwt.2006.10.008. - DOI
-
- Edwards WP. Royal Society of Chemistry. 2007. The science of sugar confectionery.
-
- Brown ZK, Fryer PJ, Norton IT, Bridson RH, Supercrit J. Fluids 54. Numb. 2010;1:89–95.
LinkOut - more resources
Full Text Sources
Other Literature Sources
