Prevalence and Risk Factors of Atypical Femoral Fracture Bone Scintigraphic Feature in Patients Experiencing Bisphosphonate-Related Osteonecrosis of the Jaw
- PMID: 30100944
- PMCID: PMC6066490
- DOI: 10.1007/s13139-018-0533-x
Prevalence and Risk Factors of Atypical Femoral Fracture Bone Scintigraphic Feature in Patients Experiencing Bisphosphonate-Related Osteonecrosis of the Jaw
Abstract
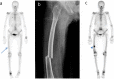
Purpose: Bisphosphonate (BP) is the first-line therapy for the management of osteoporosis. BP-related osteonecrosis of the jaw (BRONJ) and atypical femoral fracture (AFF) are increasingly common comorbidities in patients with osteoporosis under long-term BP treatment. The aim of this study was to evaluate the incidence and risk factors for AFF features on bone scintigraphy in patients with BRONJ.
Methods: Among total of 373 BRONJ patients treated between September 2005 and July 2014, 237 (220 women, 17 men; median age 73 years) who underwent three-phase bone scintigraphy were enrolled for this retrospective study. AFF features on bone scintigraphy and the related clinical factors were assessed.
Results: Among 237 patients with BRONJ, 11 (4.6%) showed AFF features on bone scintigraphy. BP medication duration (p = 0.049) correlated significantly with AFF features on bone scintigraphy in patients with BRONJ. BP intake duration of 34 months was the cutoff value for predicting the presence of AFF features on bone scintigraphy. Among the patients with BRONJ, all those with AFF features on bone scintigraphy were female patients with osteoporosis who were on oral BP medication; however, these factors were not significantly different along with AFF features on bone scintigraphy.
Conclusion: The incidence of AFF features on bone scintigraphy was relatively high in patients with BRONJ. A careful observation of patients presenting with the AFF features on bone scintigraphy may be needed, particularly for female BRONJ patients with osteoporosis who have been on BP medication for over 34 months.
Keywords: Atypical femoral fracture; Bisphosphonate-related osteonecrosis of the jaw; Bone scintigraphy; Prevalence.
Conflict of interest statement
Compliance with Ethical StandardsSeung Hyun Son, Chae Moon Hong, Ju Hye Jeong, Shin Young Jeong, Sang-Woo Lee, Jaetae Lee, Tae-Geon Kwon, and Byeong-Cheol Ahn declare that they have no conflict of interest.The study protocol had been approved by the Ethics Committee of the Kyungpook National University Hospital (KNUH 2018–02-029). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments. No informed consent was needed, because of the retrospective design of our study.The institutional review board of our institute approved this retrospective study, and the requirement to obtain informed consent was waived.
Figures

References
-
- Reid DM, Devogelaer JP, Saag K, Roux C, Lau CS, Reginster JY, et al. Zoledronic acid and risedronate in the prevention and treatment of glucocorticoid-induced osteoporosis (HORIZON): a multicentre, double-blind, double-dummy randomised controlled trial. Lancet. 2009;373:1253–1263. doi: 10.1016/S0140-6736(09)60250-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
