cAMP-mediated autophagy inhibits DNA damage-induced death of leukemia cells independent of p53
- PMID: 30100998
- PMCID: PMC6084393
- DOI: 10.18632/oncotarget.25758
cAMP-mediated autophagy inhibits DNA damage-induced death of leukemia cells independent of p53
Abstract
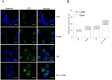
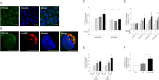
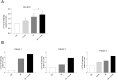
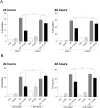
Autophagy is important in regulating the balance between cell death and survival, with the tumor suppressor p53 as one of the key components in this interplay. We have previously utilized an in vitro model of the most common form of childhood cancer, B cell precursor acute lymphoblastic leukemia (BCP-ALL), to show that activation of the cAMP signaling pathway inhibits p53-mediated apoptosis in response to DNA damage in both cell lines and primary leukemic cells. The present study reveals that cAMP-mediated survival of BCP-ALL cells exposed to DNA damaging agents, involves a critical and p53-independent enhancement of autophagy. Although autophagy generally is regarded as a survival mechanism, DNA damage-induced apoptosis has been linked both to enhanced and reduced levels of autophagy. Here we show that exposure of BCP-ALL cells to irradiation or cytotoxic drugs triggers autophagy and cell death in a p53-dependent manner. Stimulation of the cAMP signaling pathway further augments autophagy and inhibits the DNA damage-induced cell death concomitant with reduced nuclear levels of p53. Knocking-down the levels of p53 reduced the irradiation-induced autophagy and cell death, but had no effect on the cAMP-mediated autophagy. Moreover, prevention of autophagy by bafilomycin A1 or by the ULK-inhibitor MRT68921, diminished the protecting effect of cAMP signaling on DNA damage-induced cell death. Having previously proposed a role of the cAMP signaling pathway in development and treatment of BCP-ALLs, we here suggest that inhibitors of autophagy may improve current DNA damage-based therapy of BCP-ALL - independent of p53.
Keywords: DNA damage; apoptosis; autophagy; cAMP-signaling; p53.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that there are no competing financial interests in relation to the work described.
Figures





Similar articles
-
Bone marrow stroma-derived PGE2 protects BCP-ALL cells from DNA damage-induced p53 accumulation and cell death.Mol Cancer. 2015 Jan 27;14(1):14. doi: 10.1186/s12943-014-0278-9. Mol Cancer. 2015. PMID: 25623255 Free PMC article.
-
Cyclic AMP-induced p53 destabilization is independent of EPAC in pre-B acute lymphoblastic leukemia cells in vitro.J Recept Signal Transduct Res. 2011 Jun;31(3):256-63. doi: 10.3109/10799893.2011.578140. J Recept Signal Transduct Res. 2011. PMID: 21619452
-
Selective inhibition of cell death in malignant vs normal B-cell precursors: implications for cAMP in development and treatment of BCP-ALL.Blood. 2013 Mar 7;121(10):1805-13. doi: 10.1182/blood-2012-08-452698. Epub 2013 Jan 8. Blood. 2013. PMID: 23299313
-
p53-Mediated Tumor Suppression: DNA-Damage Response and Alternative Mechanisms.Cancers (Basel). 2019 Dec 9;11(12):1983. doi: 10.3390/cancers11121983. Cancers (Basel). 2019. PMID: 31835405 Free PMC article. Review.
-
p53-Autophagy-Metastasis Link.Cancers (Basel). 2018 May 18;10(5):148. doi: 10.3390/cancers10050148. Cancers (Basel). 2018. PMID: 29783720 Free PMC article. Review.
Cited by
-
Is autophagy induction by PARP inhibitors a target for therapeutic benefit?Oncol Res. 2022 Dec 6;30(1):1-12. doi: 10.32604/or.2022.026459. eCollection 2022. Oncol Res. 2022. PMID: 37304006 Free PMC article. Review.
-
Identification of an autophagy-related gene signature for predicting prognosis and immune activity in pancreatic adenocarcinoma.Sci Rep. 2022 Apr 29;12(1):7006. doi: 10.1038/s41598-022-11050-w. Sci Rep. 2022. PMID: 35488119 Free PMC article.
-
Autophagy, molecular chaperones, and unfolded protein response as promoters of tumor recurrence.Cancer Metastasis Rev. 2023 Mar;42(1):217-254. doi: 10.1007/s10555-023-10085-3. Epub 2023 Feb 1. Cancer Metastasis Rev. 2023. PMID: 36723697 Review.
-
A Syx-RhoA-Dia1 signaling axis regulates cell cycle progression, DNA damage, and therapy resistance in glioblastoma.JCI Insight. 2023 Jul 10;8(13):e157491. doi: 10.1172/jci.insight.157491. JCI Insight. 2023. PMID: 37427593 Free PMC article.
-
Resveratrol protects intestinal epithelial cells against radiation-induced damage by promoting autophagy and inhibiting apoptosis through SIRT1 activation.J Radiat Res. 2021 Jul 10;62(4):574-581. doi: 10.1093/jrr/rrab035. J Radiat Res. 2021. PMID: 33912959 Free PMC article.
References
-
- Galluzzi L, Pietrocola F, Bravo-San Pedro JM, Amaravadi RK, Baehrecke EH, Cecconi F, Codogno P, Debnath J, Gewirtz DA, Karantza V, Kimmelman A, Kumar S, Levine B, et al. Autophagy in malignant transformation and cancer progression. EMBO J. 2015;34:856–80. https://doi.org/10.15252/embj.201490784. - DOI - PMC - PubMed
-
- Chen C, Lu L, Yan S, Yi H, Yao H, Wu D, He G, Tao X, Deng X. Autophagy and doxorubicin resistance in cancer. Anticancer Drugs. 2018;29:1–9. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

