Health Economic Evaluations of Cancer in Brazil: A Systematic Review
- PMID: 30101142
- PMCID: PMC6072849
- DOI: 10.3389/fpubh.2018.00205
Health Economic Evaluations of Cancer in Brazil: A Systematic Review
Abstract
Background: A large number of health economic evaluation (HEE) studies have been published in developed countries. However, Brazilian HEE literature in oncology has not been studied.
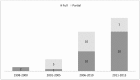
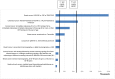
Objective: To investigate whether the scientific literature has provided a set of HEE in oncology capable of supporting decision making in the Brazilian context. Methods: A systematic review was conducted to identify and characterize studies in this field. We searched multiple databases selecting partial and full HEE studies in oncology (1998-2013). Results: Fifty-five articles were reviewed, of these, 33 (60%) were full health economic evaluations. Type of cancers most frequently studied were: breast (38.2%), cervical (14.6%), lung (10.9%) and colorectal (9.1%). Procedures (47.3%) were the technologies most frequently evaluated. In terms of the intended purposes of the technologies, most (63.6%) were treatments. The majority of the incremental cost-effectiveness ratios (ICERs) reported have been below the cost-effectiveness threshold suggested by the World Health Organization (WHO). Conclusions: There has been an increase in the number of HEEs related to cancer in Brazil. These studies may support decision-making processes regarding the coverage of and reimbursement of healthcare technologies for cancer treatment in Brazil.
Keywords: Brazil; cost-benefit analysis; costs and cost analysis; health care costs; neoplasms.
Figures
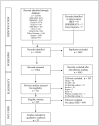

References
-
- Instituto Nacional de Câncer José Alencar Gomes da Silva (INCA) Estimativa 2018: Incidência de Câncer no Brasil. Coordenação de Prevenção e Vigilância (2017). Available online at: http://www2.inca.gov.br/wps/wcm/connect/en-inca/portal/home
-
- American Society of Clinical Oncology (ASCO) Major Cancer Milestones. American Society of Clinical Oncology. (2014). Available online at: https://www.asco.org/research-progress/cancer-progress-timeline
-
- Drummond M, Sculpher M, Torrance G, O'Brien B SG. Methods for the Economic Evaluation of Health Care Programmes. New York, NY: Oxford University Press; (2015).
LinkOut - more resources
Full Text Sources
Other Literature Sources

