Genetically Corrected iPSC-Derived Neural Stem Cell Grafts Deliver Enzyme Replacement to Affect CNS Disease in Sanfilippo B Mice
- PMID: 30101150
- PMCID: PMC6076361
- DOI: 10.1016/j.omtm.2018.06.005
Genetically Corrected iPSC-Derived Neural Stem Cell Grafts Deliver Enzyme Replacement to Affect CNS Disease in Sanfilippo B Mice
Abstract
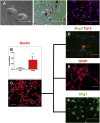
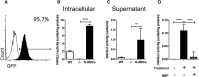
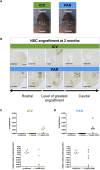
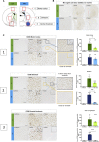
Sanfilippo syndrome type B (mucopolysaccharidosis type IIIB [MPS IIIB]) is a lysosomal storage disorder primarily affecting the brain that is caused by a deficiency in the enzyme α-N-acetylglucosaminidase (NAGLU), leading to intralysosomal accumulation of heparan sulfate. There are currently no treatments for this disorder. Here we report that, ex vivo, lentiviral correction of Naglu-/- neural stem cells derived from Naglu-/- mice (iNSCs) corrected their lysosomal pathology and allowed them to secrete a functional NAGLU enzyme that could be taken up by deficient cells. Following long-term transplantation of these corrected iNSCs into Naglu-/- mice, we detected NAGLU activity in the majority of engrafted animals. Successfully transplanted Naglu-/- mice showed a significant decrease in storage material, a reduction in astrocyte activation, and complete prevention of microglial activation within the area of engrafted cells and neighboring regions, with beneficial effects extending partway along the rostrocaudal axis of the brain. Our results demonstrate long-term engraftment of iNSCs in the brain that are capable of cross-correcting pathology in Naglu-/- mice. Our findings suggest that genetically engineered iNSCs could potentially be used to deliver enzymes and treat MPS IIIB.
Keywords: MPS IIIB; lysosomal storage disorder; stem cell therapy.
Figures


Similar articles
-
Brain transplantation of genetically corrected Sanfilippo type B neural stem cells induces partial cross-correction of the disease.Mol Ther Methods Clin Dev. 2022 Oct 27;27:452-463. doi: 10.1016/j.omtm.2022.10.013. eCollection 2022 Dec 8. Mol Ther Methods Clin Dev. 2022. PMID: 36419468 Free PMC article.
-
Macrophage enzyme and reduced inflammation drive brain correction of mucopolysaccharidosis IIIB by stem cell gene therapy.Brain. 2018 Jan 1;141(1):99-116. doi: 10.1093/brain/awx311. Brain. 2018. PMID: 29186350
-
Clearance of Heparan Sulfate and Attenuation of CNS Pathology by Intracerebroventricular BMN 250 in Sanfilippo Type B Mice.Mol Ther Methods Clin Dev. 2017 Jun 6;6:43-53. doi: 10.1016/j.omtm.2017.05.009. eCollection 2017 Sep 15. Mol Ther Methods Clin Dev. 2017. PMID: 28664165 Free PMC article.
-
Molecular genetics of mucopolysaccharidosis type IIIA and IIIB: Diagnostic, clinical, and biological implications.Hum Mutat. 2001 Oct;18(4):264-81. doi: 10.1002/humu.1189. Hum Mutat. 2001. PMID: 11668611 Review.
-
Heparan Sulfate, Mucopolysaccharidosis IIIB and Sulfur Metabolism Disorders.Antioxidants (Basel). 2022 Mar 30;11(4):678. doi: 10.3390/antiox11040678. Antioxidants (Basel). 2022. PMID: 35453363 Free PMC article. Review.
Cited by
-
Biochemical evaluation of intracerebroventricular rhNAGLU-IGF2 enzyme replacement therapy in neonatal mice with Sanfilippo B syndrome.Mol Genet Metab. 2021 Jun;133(2):185-192. doi: 10.1016/j.ymgme.2021.03.013. Epub 2021 Mar 31. Mol Genet Metab. 2021. PMID: 33839004 Free PMC article.
-
Mucopolysaccharidosis III: Molecular basis and treatment.Pediatr Endocrinol Diabetes Metab. 2021;27(3):201-208. doi: 10.5114/pedm.2021.109270. Pediatr Endocrinol Diabetes Metab. 2021. PMID: 34743503 Free PMC article. Review.
-
Enzyme Replacement Therapy for Mucopolysaccharidosis IIID using Recombinant Human α-N-Acetylglucosamine-6-Sulfatase in Neonatal Mice.Mol Pharm. 2021 Jan 4;18(1):214-227. doi: 10.1021/acs.molpharmaceut.0c00831. Epub 2020 Dec 15. Mol Pharm. 2021. PMID: 33320673 Free PMC article.
-
Neurological Disease Modeling Using Pluripotent and Multipotent Stem Cells: A Key Step towards Understanding and Treating Mucopolysaccharidoses.Biomedicines. 2023 Apr 21;11(4):1234. doi: 10.3390/biomedicines11041234. Biomedicines. 2023. PMID: 37189853 Free PMC article. Review.
-
Brain transplantation of genetically corrected Sanfilippo type B neural stem cells induces partial cross-correction of the disease.Mol Ther Methods Clin Dev. 2022 Oct 27;27:452-463. doi: 10.1016/j.omtm.2022.10.013. eCollection 2022 Dec 8. Mol Ther Methods Clin Dev. 2022. PMID: 36419468 Free PMC article.
References
-
- Valstar M.J., Ruijter G.J., van Diggelen O.P., Poorthuis B.J., Wijburg F.A. Sanfilippo syndrome: a mini-review. J. Inherit. Metab. Dis. 2008;31:240–252. - PubMed
-
- Andrade F., Aldámiz-Echevarría L., Llarena M., Couce M.L. Sanfilippo syndrome: Overall review. Pediatr. Int. 2015;57:331–338. - PubMed
-
- Kakkis E.D., Muenzer J., Tiller G.E., Waber L., Belmont J., Passage M., Izykowski B., Phillips J., Doroshow R., Walot I. Enzyme-replacement therapy in mucopolysaccharidosis I. N. Engl. J. Med. 2001;344:182–188. - PubMed
-
- Muenzer J., Gucsavas-Calikoglu M., McCandless S.E., Schuetz T.J., Kimura A. A phase I/II clinical trial of enzyme replacement therapy in mucopolysaccharidosis II (Hunter syndrome) Mol. Genet. Metab. 2007;90:329–337. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

