A Bovine Adenoviral Vector-Based H5N1 Influenza -Vaccine Provides Enhanced Immunogenicity and Protection at a Significantly Low Dose
- PMID: 30101154
- PMCID: PMC6082999
- DOI: 10.1016/j.omtm.2018.07.007
A Bovine Adenoviral Vector-Based H5N1 Influenza -Vaccine Provides Enhanced Immunogenicity and Protection at a Significantly Low Dose
Abstract
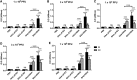
Several human and nonhuman adenovirus (AdV) vectors including bovine AdV type 3 (BAdV-3) were developed as gene delivery vectors to supplement and/or elude human AdV (HAdV)-specific neutralizing antibodies (vector immunity). Here we evaluated the vaccine immunogenicity and efficacy of BAdV-3 vector (BAd-H5HA) expressing hemagglutinin (HA) of a H5N1 influenza virus in a dose escalation study in mice with the intranasal (IN) or intramuscular (IM) route of inoculation in comparison with the HAdV type C5 (HAdV-C5) vector (HAd-H5HA) expressing HA of a H5N1 influenza virus. Dose-related increases in the immune responses were clearly noticeable. A single IM inoculation with BAd-H5HA resulted in enhanced cellular immune responses compared with that of HAd-H5HA and conferred complete protection following challenge with a heterologous H5N1 virus at the dose of 3 × 107 plaque-forming units (PFUs), whereas a significant amount of influenza virus was detected in the lungs of mice immunized with 1 × 108 PFUs of HAd-H5HA. Similarly, compared with that of HAd-H5HA, a single IN inoculation with BAd-H5HA produced significantly enhanced humoral (HA-specific immunoglobulin [IgG] and its subclasses, as well as HA-specific IgA) and cellular immune responses, and conferred complete protection following challenge with a heterologous H5N1 virus. Complete protection with BAd-H5HA was observed with the lowest vaccine dose (1 × 106 PFUs), but similar protection with HAd-H5HA was observed at the highest vaccine dose (1 × 108 PFUs). These results suggest that at least 30-fold dose sparing can be achieved with BAd-H5HA vector compared with HAd-H5HA vaccine vector.
Keywords: adenoviral vector; avian influenza; bovine adenoviral vector; dose sparing; enhanced immunogenicity and protection; human adenoviral vector; route of immunization; universal influenza vaccine.
Figures




Similar articles
-
Enhancement of mucosal innate and adaptive immunity following intranasal immunization of mice with a bovine adenoviral vector.Front Immunol. 2023 Nov 21;14:1305937. doi: 10.3389/fimmu.2023.1305937. eCollection 2023. Front Immunol. 2023. PMID: 38077380 Free PMC article.
-
Bovine adenoviral vector-based H5N1 influenza vaccine overcomes exceptionally high levels of pre-existing immunity against human adenovirus.Mol Ther. 2008 May;16(5):965-71. doi: 10.1038/mt.2008.12. Epub 2008 Feb 26. Mol Ther. 2008. PMID: 18301400 Free PMC article.
-
Longevity of adenovirus vector immunity in mice and its implications for vaccine efficacy.Vaccine. 2018 Oct 29;36(45):6744-6751. doi: 10.1016/j.vaccine.2018.09.031. Epub 2018 Sep 25. Vaccine. 2018. PMID: 30266488 Free PMC article.
-
Progress on adenovirus-vectored universal influenza vaccines.Hum Vaccin Immunother. 2015;11(5):1209-22. doi: 10.1080/21645515.2015.1016674. Hum Vaccin Immunother. 2015. PMID: 25876176 Free PMC article. Review.
-
Adenoviral Vector-Based Vaccine Platforms for Developing the Next Generation of Influenza Vaccines.Vaccines (Basel). 2020 Oct 1;8(4):574. doi: 10.3390/vaccines8040574. Vaccines (Basel). 2020. PMID: 33019589 Free PMC article. Review.
Cited by
-
A bovine adenoviral-vector-based universal influenza vaccine confers protection against influenza A and B viruses in mice and ferrets.Mol Ther Nucleic Acids. 2025 Jun 9;36(3):102594. doi: 10.1016/j.omtn.2025.102594. eCollection 2025 Sep 9. Mol Ther Nucleic Acids. 2025. PMID: 40635672 Free PMC article.
-
Nonhuman Adenoviral Vector-Based Platforms and Their Utility in Designing Next Generation of Vaccines for Infectious Diseases.Viruses. 2021 Jul 29;13(8):1493. doi: 10.3390/v13081493. Viruses. 2021. PMID: 34452358 Free PMC article. Review.
-
Influenza A in Bovine Species: A Narrative Literature Review.Viruses. 2019 Jun 17;11(6):561. doi: 10.3390/v11060561. Viruses. 2019. PMID: 31213032 Free PMC article. Review.
-
Adenoviral Vectors as Vaccines for Emerging Avian Influenza Viruses.Front Immunol. 2021 Jan 29;11:607333. doi: 10.3389/fimmu.2020.607333. eCollection 2020. Front Immunol. 2021. PMID: 33633727 Free PMC article. Review.
-
Adenoviral Vector-Based Vaccine Expressing Hemagglutinin Stem Region with Autophagy-Inducing Peptide Confers Cross-Protection Against Group 1 and 2 Influenza A Viruses.Vaccines (Basel). 2025 Jan 20;13(1):95. doi: 10.3390/vaccines13010095. Vaccines (Basel). 2025. PMID: 39852874 Free PMC article.
References
-
- Bangari D.S., Mittal S.K. Porcine adenoviral vectors evade preexisting humoral immunity to adenoviruses and efficiently infect both human and murine cells in culture. Virus Res. 2004;105:127–136. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

