Microglial pannexin-1 channel activation is a spinal determinant of joint pain
- PMID: 30101191
- PMCID: PMC6082646
- DOI: 10.1126/sciadv.aas9846
Microglial pannexin-1 channel activation is a spinal determinant of joint pain
Abstract
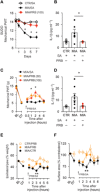
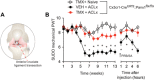
Chronic joint pain such as mechanical allodynia is the most debilitating symptom of arthritis, yet effective therapies are lacking. We identify the pannexin-1 (Panx1) channel as a therapeutic target for alleviating mechanical allodynia, a cardinal sign of arthritis. In rats, joint pain caused by intra-articular injection of monosodium iodoacetate (MIA) was associated with spinal adenosine 5'-triphosphate (ATP) release and a microglia-specific up-regulation of P2X7 receptors (P2X7Rs). Blockade of P2X7R or ablation of spinal microglia prevented and reversed mechanical allodynia. P2X7Rs drive Panx1 channel activation, and in rats with mechanical allodynia, Panx1 function was increased in spinal microglia. Specifically, microglial Panx1-mediated release of the proinflammatory cytokine interleukin-1β (IL-1β) induced mechanical allodynia in the MIA-injected hindlimb. Intrathecal administration of the Panx1-blocking peptide 10panx suppressed the aberrant discharge of spinal laminae I-II neurons evoked by innocuous mechanical hindpaw stimulation in arthritic rats. Furthermore, mice with a microglia-specific genetic deletion of Panx1 were protected from developing mechanical allodynia. Treatment with probenecid, a clinically used broad-spectrum Panx1 blocker, resulted in a striking attenuation of MIA-induced mechanical allodynia and normalized responses in the dynamic weight-bearing test, without affecting acute nociception. Probenecid reversal of mechanical allodynia was also observed in rats 13 weeks after anterior cruciate ligament transection, a model of posttraumatic osteoarthritis. Thus, Panx1-targeted therapy is a new mechanistic approach for alleviating joint pain.
Figures
Similar articles
-
Blocking microglial pannexin-1 channels alleviates morphine withdrawal in rodents.Nat Med. 2017 Mar;23(3):355-360. doi: 10.1038/nm.4281. Epub 2017 Jan 30. Nat Med. 2017. PMID: 28134928
-
Pannexin 1: a novel participant in neuropathic pain signaling in the rat spinal cord.Pain. 2014 Oct;155(10):2108-15. doi: 10.1016/j.pain.2014.07.024. Epub 2014 Aug 4. Pain. 2014. PMID: 25102401
-
Divergent sex-specific pannexin-1 mechanisms in microglia and T cells underlie neuropathic pain.Neuron. 2025 Mar 19;113(6):896-911.e9. doi: 10.1016/j.neuron.2025.01.005. Epub 2025 Jan 31. Neuron. 2025. PMID: 39892387
-
Interactions of pannexin 1 with NMDA and P2X7 receptors in central nervous system pathologies: Possible role on chronic pain.Pharmacol Res. 2015 Nov;101:86-93. doi: 10.1016/j.phrs.2015.07.016. Epub 2015 Jul 23. Pharmacol Res. 2015. PMID: 26211949 Review.
-
Revisiting multimodal activation and channel properties of Pannexin 1.J Gen Physiol. 2018 Jan 2;150(1):19-39. doi: 10.1085/jgp.201711888. Epub 2017 Dec 12. J Gen Physiol. 2018. PMID: 29233884 Free PMC article. Review.
Cited by
-
Pannexin-1 Channels as Mediators of Neuroinflammation.Int J Mol Sci. 2021 May 14;22(10):5189. doi: 10.3390/ijms22105189. Int J Mol Sci. 2021. PMID: 34068881 Free PMC article. Review.
-
Intrathecal injection of brilliant blue G, a P2X7 antagonist, attenuates the exercise pressor reflex in rats.Am J Physiol Regul Integr Comp Physiol. 2020 Aug 1;319(2):R223-R232. doi: 10.1152/ajpregu.00093.2020. Epub 2020 Jul 1. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 32609538 Free PMC article.
-
Neuropathic pain; what we know and what we should do about it.Front Pain Res (Lausanne). 2023 Sep 22;4:1220034. doi: 10.3389/fpain.2023.1220034. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 37810432 Free PMC article. Review.
-
The Similar and Distinct Roles of Satellite Glial Cells and Spinal Astrocytes in Neuropathic Pain.Cells. 2023 Mar 22;12(6):965. doi: 10.3390/cells12060965. Cells. 2023. PMID: 36980304 Free PMC article. Review.
-
Sensory neuron-specific block of multifaceted sodium channels mitigates neuropathic pain behaviors of osteoarthritis.Pain Rep. 2025 May 27;10(4):e1288. doi: 10.1097/PR9.0000000000001288. eCollection 2025 Aug. Pain Rep. 2025. PMID: 40444021 Free PMC article.
References
-
- Hannan M. T., Felson D. T., Pincus T., Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J. Rheumatol. 27, 1513–1517 (2000). - PubMed
-
- Gwilym S. E., Keltner J. R., Warnaby C. E., Carr A. J., Chizh B., Chessell I., Tracey I., Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 61, 1226–1234 (2009). - PubMed
-
- Sagar D. R., Burston J. J., Hathway G. J., Woodhams S. G., Pearson R. G., Bennett A. J., Kendall D. A., Scammell B. E., Chapman V., Sagar D. R., Burston J. J., Hathway G. J., Woodhams S. G., Pearson R. G., Bennett A. J., Kendall D. A., Scammell B. E., Chapman V., The contribution of spinal glial cells to chronic pain behaviour in the monosodium iodoacetate model of osteoarthritic pain. Mol. Pain 7, 88 (2011). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

