Hypoglycemia After Gastric Bypass Surgery: Current Concepts and Controversies
- PMID: 30101281
- PMCID: PMC6692713
- DOI: 10.1210/jc.2018-00528
Hypoglycemia After Gastric Bypass Surgery: Current Concepts and Controversies
Abstract
Context: Hypoglycemia, occurring after bariatric and other forms of upper gastrointestinal surgery, is increasingly encountered by clinical endocrinologists. The true frequency of this condition remains uncertain, due, in part, to differences in the diagnostic criteria and in the affected populations, as well as relative lack of patient and physician awareness and understanding of this condition. Postbariatric hypoglycemia can be severe and disabling for some patients, with neuroglycopenia (altered cognition, seizures, and loss of consciousness) leading to falls, motor vehicle accidents, and job and income loss. Moreover, repeated episodes of hypoglycemia can result in hypoglycemia unawareness, further impairing safety and requiring the assistance of others to treat hypoglycemia.
Objective: In this review, we summarize and integrate data from studies of patients affected by hypoglycemia after Roux-en-Y gastric bypass (RYGB) surgery, obtained from PubMed searches (1990 to 2017) and reference searches of relevant retrieved articles. Whereas hypoglycemia can also be observed after sleeve gastrectomy and fundoplication, this review is focused on post-RYGB, given the greater body of published clinical studies at present.
Outcome measures: Data addressing specific aspects of diagnosis, pathophysiology, and treatment were reviewed by the authors; when not available, the authors have provided opinions based on clinical experience with this challenging condition.
Conclusions: Hypoglycemia, occurring after gastric bypass surgery, is challenging for patients and physicians alike. This review provides a systematic approach to diagnosis and treatment based on the underlying pathophysiology.
Figures
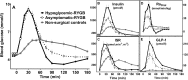


References
-
- Whipple AO. The surgical therapy of hyperisulinism. J Int Chir. 1938;3:237–276.
-
- Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ; Endocrine Society . Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709–728. - PubMed
-
- Basu A, Service FJ, Yu L, Heser D, Ferries LM, Eisenbarth G. Insulin autoimmunity and hypoglycemia in seven white patients. Endocr Pract. 2005;11(2):97–103. - PubMed
-
- Tack J, Arts J, Caenepeel P, De Wulf D, Bisschops R. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat Rev Gastroenterol Hepatol. 2009;6(10):583–590. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

