A novel protocol for the preparation of active recombinant human pancreatic lipase from Escherichia coli
- PMID: 30101295
- PMCID: PMC6267337
- DOI: 10.1093/jb/mvy067
A novel protocol for the preparation of active recombinant human pancreatic lipase from Escherichia coli
Abstract
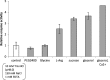
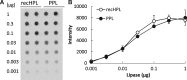
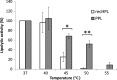
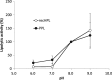
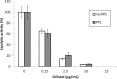
An active recombinant human pancreatic lipase (recHPL) was successfully prepared for the first time from the Escherichia coli expression system using short Strep-tag II (ST II). The recHPL-ST II was solubilized using 8 M urea from E.coli lysate and purified on a Strep-Tactin-Sepharose column. After refolding by stepwise dialyses in the presence of glycerol and Ca2+ for 2 days followed by gel filtration, 1.8-6 mg of active recHPL-ST II was obtained from 1 L of culture. The recHPL was non-glycosylated, but showed almost equal specific activity, pH-dependency and time-dependent stability compared to those of native porcine pancreatic lipase (PPL) at 37°C. However, the recHPL lost its lipolytic activity above 50°C, showing a lower heat-stability than that of native PPL, which retained half its activity at this temperature.
Figures


Similar articles
-
Preparation and Purification of Active Recombinant Human Pancreatic Lipase in Escherichia coli.Bio Protoc. 2019 Jul 5;9(13):e3286. doi: 10.21769/BioProtoc.3286. eCollection 2019 Jul 5. Bio Protoc. 2019. PMID: 33654801 Free PMC article.
-
High-level expression of lipase in Escherichia coli and recovery of active recombinant enzyme through in vitro refolding.Protein Expr Purif. 2010 Mar;70(1):75-80. doi: 10.1016/j.pep.2009.08.009. Epub 2009 Aug 27. Protein Expr Purif. 2010. PMID: 19716421
-
Purification, refolding, and characterization of recombinant Pseudomonas fluorescens lipase.Protein Expr Purif. 2005 Jan;39(1):124-9. doi: 10.1016/j.pep.2004.09.014. Protein Expr Purif. 2005. PMID: 15596368
-
Co-expression of the lipase and foldase of Pseudomonas aeruginosa to a functional lipase in Escherichia coli.Appl Microbiol Biotechnol. 2010 Jan;85(3):597-604. doi: 10.1007/s00253-009-2131-4. Epub 2009 Jul 21. Appl Microbiol Biotechnol. 2010. PMID: 19629472
-
Folding of eukaryotic proteins produced in Escherichia coli.Genet Eng (N Y). 1990;12:1-19. doi: 10.1007/978-1-4613-0641-2_1. Genet Eng (N Y). 1990. PMID: 1366701 Review.
Cited by
-
The Role of Calcium Ions in Restoring Lipase Activity of Recombinant Human Lipoprotein Lipase Expressed in Bacteria.Mol Biotechnol. 2025 Apr 24. doi: 10.1007/s12033-025-01440-6. Online ahead of print. Mol Biotechnol. 2025. PMID: 40274713
-
Recent Advances in Overexpression of Functional Recombinant Lipases.Mol Biotechnol. 2023 Nov;65(11):1737-1749. doi: 10.1007/s12033-023-00725-y. Epub 2023 Mar 27. Mol Biotechnol. 2023. PMID: 36971996 Review.
-
Preparation and Purification of Active Recombinant Human Pancreatic Lipase in Escherichia coli.Bio Protoc. 2019 Jul 5;9(13):e3286. doi: 10.21769/BioProtoc.3286. eCollection 2019 Jul 5. Bio Protoc. 2019. PMID: 33654801 Free PMC article.
References
-
- Bray G.A. (2004) Medical consequences of obesity. J. Clin. Endocrinol. Metab. 89, 2583–2589 - PubMed
-
- Hermoso J., Pignol D., Kerfelec B., Crenon I., Chapus C., Fontecilla-Camps J.C. (1996) Lipase activation by nonionic detergents. The crystal structure of the porcine lipase-colipase-tetraethylene glycol monooctyl ether complex. J. Biol. Chem. 271, 18007–18016 - PubMed
-
- De Caro A., Figarella C., Amic J., Michel R., Guy O. (1977) Human pancreatic lipase: a glycoprotein. Biochim. Biophys. Acta 490, 411–419 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

