Opposing roles of CB1 and CB2 cannabinoid receptors in the stimulant and rewarding effects of cocaine
- PMID: 30101419
- PMCID: PMC6487550
- DOI: 10.1111/bph.14473
Opposing roles of CB1 and CB2 cannabinoid receptors in the stimulant and rewarding effects of cocaine
Abstract
Background and purpose: The endocannabinoids anandamide and 2-arachidonoylglycerol (2-AG) bind to CB1 and CB2 cannabinoid receptors in the brain and modulate the mesolimbic dopaminergic pathway. This neurocircuitry is engaged by psychostimulant drugs, including cocaine. Although CB1 receptor antagonism and CB2 receptor activation are known to inhibit certain effects of cocaine, they have been investigated separately. Here, we tested the hypothesis that there is a reciprocal interaction between CB1 receptor blockade and CB2 receptor activation in modulating behavioural responses to cocaine.
Experimental approach: Male Swiss mice received i.p. injections of cannabinoid-related drugs followed by cocaine, and were then tested for cocaine-induced hyperlocomotion, c-Fos expression in the nucleus accumbens and conditioned place preference. Levels of endocannabinoids after cocaine injections were also analysed.
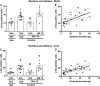
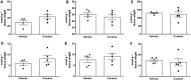
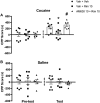
Key results: The CB1 receptor antagonist, rimonabant, and the CB2 receptor agonist, JWH133, prevented cocaine-induced hyperlocomotion. The same results were obtained by combining sub-effective doses of both compounds. The CB2 receptor antagonist, AM630, reversed the inhibitory effects of rimonabant in cocaine-induced hyperlocomotion and c-Fos expression in the nucleus accumbens. Selective inhibitors of anandamide and 2-AG hydrolysis (URB597 and JZL184, respectively) failed to modify this response. However, JZL184 prevented cocaine-induced hyperlocomotion when given after a sub-effective dose of rimonabant. Cocaine did not change brain endocannabinoid levels. Finally, CB2 receptor blockade reversed the inhibitory effect of rimonabant in the acquisition of cocaine-induced conditioned place preference.
Conclusion and implications: The present data support the hypothesis that CB1 and CB2 receptors work in concert with opposing functions to modulate certain addiction-related effects of cocaine.
Linked articles: This article is part of a themed section on 8th European Workshop on Cannabinoid Research. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v176.10/issuetoc.
© 2018 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Attenuation of Cocaine-Induced Conditioned Place Preference and Motor Activity via Cannabinoid CB2 Receptor Agonism and CB1 Receptor Antagonism in Rats.Int J Neuropsychopharmacol. 2017 Mar 1;20(3):269-278. doi: 10.1093/ijnp/pyw102. Int J Neuropsychopharmacol. 2017. PMID: 27994006 Free PMC article.
-
2-AG promotes the expression of conditioned fear via cannabinoid receptor type 1 on GABAergic neurons.Psychopharmacology (Berl). 2015 Aug;232(15):2811-25. doi: 10.1007/s00213-015-3917-y. Epub 2015 Mar 28. Psychopharmacology (Berl). 2015. PMID: 25814137
-
The roles of cannabinoid CB1 and CB2 receptors in cocaine-induced behavioral sensitization and conditioned place preference in mice.Psychopharmacology (Berl). 2020 Feb;237(2):385-394. doi: 10.1007/s00213-019-05370-5. Epub 2019 Oct 30. Psychopharmacology (Berl). 2020. PMID: 31667531
-
Why do cannabinoid receptors have more than one endogenous ligand?Philos Trans R Soc Lond B Biol Sci. 2012 Dec 5;367(1607):3216-28. doi: 10.1098/rstb.2011.0382. Philos Trans R Soc Lond B Biol Sci. 2012. PMID: 23108541 Free PMC article. Review.
-
The endocannabinoid system: Overview of an emerging multi-faceted therapeutic target.Prostaglandins Leukot Essent Fatty Acids. 2019 Jan;140:51-56. doi: 10.1016/j.plefa.2018.11.016. Epub 2018 Nov 29. Prostaglandins Leukot Essent Fatty Acids. 2019. PMID: 30553404 Review.
Cited by
-
Xie2-64, a novel CB2 receptor inverse agonist, reduces cocaine abuse-related behaviors in rodents.Neuropharmacology. 2020 Oct 1;176:108241. doi: 10.1016/j.neuropharm.2020.108241. Epub 2020 Jul 24. Neuropharmacology. 2020. PMID: 32712273 Free PMC article.
-
Adolescent cannabinoid exposure modulates the vulnerability to cocaine-induced conditioned place preference and DNMT3a expression in the prefrontal cortex in Swiss mice.Psychopharmacology (Berl). 2021 Nov;238(11):3107-3118. doi: 10.1007/s00213-021-05926-4. Epub 2021 Jul 30. Psychopharmacology (Berl). 2021. PMID: 34328516
-
Effects of the monoamine stabilizer, (-)-OSU6162, on cocaine-induced locomotion and conditioned place preference in mice.Naunyn Schmiedebergs Arch Pharmacol. 2021 Jun;394(6):1143-1152. doi: 10.1007/s00210-021-02053-x. Epub 2021 Jan 20. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 33471153
-
Hippocampal Cannabinoid 1 Receptors Are Modulated Following Cocaine Self-administration in Male Rats.Mol Neurobiol. 2022 Mar;59(3):1896-1911. doi: 10.1007/s12035-022-02722-9. Epub 2022 Jan 15. Mol Neurobiol. 2022. PMID: 35032317
-
Candidate Therapeutics by Screening for Multitargeting Ligands: Combining the CB2 Receptor With CB1, PPARγ and 5-HT4 Receptors.Front Pharmacol. 2022 Feb 28;13:812745. doi: 10.3389/fphar.2022.812745. eCollection 2022. Front Pharmacol. 2022. PMID: 35295337 Free PMC article.
References
-
- Almeida‐Santos AF, Gobira PH, Souza DP, Ferreira RC, Romero TR, Duarte ID et al (2014). The antipsychotic aripiprazole selectively prevents the stimulant and rewarding effects of morphine in mice. Eur J Pharmacol 742: 139–144. - PubMed
-
- Batista LA, Gobira PH, Viana TG, Aguiar DC, Moreira FA (2014). Inhibition of endocannabinoid neuronal uptake and hydrolysis as strategies for developing anxiolytic drugs. Behav Pharmacol 25: 425–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

