Apalutamide for the treatment of prostate cancer
- PMID: 30101644
- PMCID: PMC6643296
- DOI: 10.1080/14737140.2018.1503954
Apalutamide for the treatment of prostate cancer
Abstract
Five new agents have been shown to prolong survival in patients with metastatic castration-resistant prostate cancer, including two targeting androgen receptor signaling (abiraterone acetate plus prednisone; enzalutamide). Recognition that these tumors remain driven by androgen receptor signaling has prompted clinical evaluation of these agents at earlier states in the prostate cancer disease continuum, along with the continued development of new agents targeting this pathway. Areas covered: This article focuses on apalutamide, a next-generation nonsteroidal antiandrogen, with current literature queried in PubMed/Medline. A narrative review strategy describes studies from engineering of the compound through to a 5-year outlook. Expert commentary: In the phase III SPARTAN study, apalutamide significantly improved metastasis-free survival in patients with nonmetastatic castration-resistant prostate cancer - the first treatment approved by the US Food and Drug Administration for this indication. Phase III studies are under way to determine the clinical benefit of apalutamide in other disease states. Given the multiplicity of prostate cancer treatment options now available, there is a need to maximize individual patient benefit through the development and validation of predictive biomarkers of sensitivity to drugs that can be used in real time to determine the optimal sequence and combinations of treatments for patients in need.
Keywords: Androgen receptor inhibitors; apalutamide; efficacy; metastatic; nonmetastatic; pharmacodynamics; pharmacokinetics; prostate cancer; safety; tolerability.
Conflict of interest statement
Declaration of interest
Dana E. Rathkopf reports research funding from Janssen and uncompensated research funding from Janssen, Astellas/Medivation, Genentech/Roche, Celgene, TAIHO, Tracon, Novartis, Millenium, and Ferring. Howard I. Scher reports non-financial support as an uncompensated consultant from Aragon relevant to this review; and personal fees as a compensated consultant from Astellas and Clovis Oncology; non-financial support as an uncompensated consultant from Ferring Pharmaceuticals and Janssen Research & Development; personal fees as a compensated consultant from Merck, Sanofi Aventis, and WCG Oncology; institutional grants to Memorial Sloan Kettering Cancer Center from Illumina, Inc, Innocrin Pharma, and Janssen; and personal fees as a compensated member of the Board Of Directors from Asterias Biotherapeutics outside the submitted work.
Figures

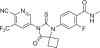



References
-
- International Agency for Research on Cancer. Prostate cancer: estimated incidence m, and prevalence worldwide GLOBOCAN Web site. [Internet]. [June 29, 2017]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
-
- SEER cancer stat facts: prostate cancer National Cancer Institute, Bethesda, MD: [Internet]. [July 6, 2017]. Available from: https://seer.cancer.gov/statfacts/html/prost.html
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68(1):7–30. - PubMed
-
- Scher HI, Heller G. Clinical states in prostate cancer: toward a dynamic model of disease progression. Urology 2000;55(3):323–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
