A Drug Eluting Scaffold for the Treatment of Arthrofibrosis
- PMID: 30101668
- PMCID: PMC6426273
- DOI: 10.1089/ten.TEC.2018.0136
A Drug Eluting Scaffold for the Treatment of Arthrofibrosis
Abstract
Introduction: The inflammatory cascade and production of prostaglandins may play a role in the pathogenesis of arthrofibrosis, a debilitating condition after joint replacement and other orthopedic procedures. Cyclooxygenase 2 (COX-2) inhibitors may mitigate the inflammatory response and formation of arthrofibrosis, but oral delivery is associated with risk of systemic side effects in many patients. The nonsteroidal anti-inflammatory drug, celecoxib, may have therapeutic benefits for arthrofibrosis, but current methods for its local delivery (e.g., biologically derived microspheres) are not translatable to immediate clinical use. Therefore, we investigated the use of a drug scaffold for sustainable intra-articular delivery of therapeutic doses of celecoxib.
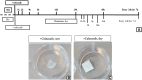
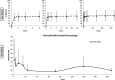
Materials and methods: Celecoxib was eluted from clinically approved biodegradable collagen membranes over 7 days as measured by UV spectroscopy and high-performance liquid chromatography/mass spectroscopy. Eluted concentrations of celecoxib were examined for toxicity (live/dead staining) and profibrotic gene expression (real-time-quantitative polymerase chain reaction) in rabbit knee capsular fibroblasts in vitro.
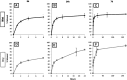
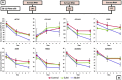
Results: Sustained concentrations of celecoxib eluted from the membrane over 7 days from both a wet and dry scaffold, with a burst release (30-45%) of celecoxib in the first 2 h. Rabbit cells treated with eluted concentrations experienced a toxic response to the burst release doses, and inhibitory effects on profibrotic genes were seen in response to the sustained doses eluted from the scaffold.
Conclusions: This study characterized the novel use of collagen scaffolds for intra-articular drug delivery to treat arthrofibrosis. Scaffolds exhibit celecoxib release through an initial burst release followed by sustained release of antifibrotic doses over 7 days. Thus, collagen scaffolds are promising for clinician-directed treatment of arthrofibrosis.
Keywords: arthrofibrosis; collagen; joint replacement.
Conflict of interest statement
No competing financial interests exist.
Figures



References
-
- Cheuy V.A., Foran J.R.H., Paxton R.J., Bade M.J., Zeni J.A., and Stevens-Lapsley J.E. Arthrofibrosis associated with total knee arthroplasty. J Arthroplast 32, 2604, 2017. - PubMed
-
- Nesterenko S., Sanchez-Sotelo J., and Morrey B.F. Refractory elbow arthrofibrosis. A report of four cases. J Bone Joint Surg Am 91, 2693, 2009. - PubMed
-
- Newman E.T., Herschmiller T.A., Attarian D.E., Vail T.P., Bolognesi M.P., and Wellman S.S. Risk factors, outcomes, and timing of manipulation under anesthesia after total knee arthroplasty. J Arthroplast 33, 245, 2018. - PubMed
-
- Janssen M., Timur U.T., Woike N., et al. . Celecoxib-loaded PEA microspheres as an auto regulatory drug-delivery system after intra-articular injection. J Control Release 244, 30, 2016. - PubMed
Appendix Reference
-
- Saha R.N., et al. Determination of celecoxib in pharmaceutical formulations using UV spectrophotometry and liquid chromatography. J Pharma and Biomed Analysis 28, 741, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

