The role of K63-linked polyubiquitination in cardiac hypertrophy
- PMID: 30102008
- PMCID: PMC6156430
- DOI: 10.1111/jcmm.13669
The role of K63-linked polyubiquitination in cardiac hypertrophy
Abstract
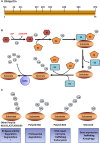
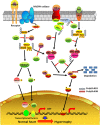
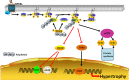
Ubiquitination, also known as ubiquitylation, is a vital post-translational modification of proteins that play a crucial role in the multiple biological processes including cell growth, proliferation and apoptosis. K63-linked ubiquitination is one of the vital post-translational modifications of proteins that are involved in the activation of protein kinases and protein trafficking during cell survival and proliferation. It also contributes to the development of various disorders including cancer, neurodegeneration and cardiac hypertrophy. In this review, we summarize the role of K63-linked ubiquitination signalling in protein kinase activation and its implications in cardiac hypertrophy. We have also provided our perspectives on therapeutically targeting K63-linked ubiquitination in downstream effector molecules of growth factor receptors for the treatment of cardiac hypertrophy.
Keywords: AKT; NF-κB; TRAF6; cardiac hypertrophy; phosphorylation; protein kinase; tumorigenesis; ubiquitination.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures
Similar articles
-
TRAF Family Member-associated NF-κB Activator (TANK) Inhibits Genotoxic Nuclear Factor κB Activation by Facilitating Deubiquitinase USP10-dependent Deubiquitination of TRAF6 Ligase.J Biol Chem. 2015 May 22;290(21):13372-85. doi: 10.1074/jbc.M115.643767. Epub 2015 Apr 10. J Biol Chem. 2015. PMID: 25861989 Free PMC article.
-
Ablation of Akt2 protects against lipopolysaccharide-induced cardiac dysfunction: role of Akt ubiquitination E3 ligase TRAF6.J Mol Cell Cardiol. 2014 Sep;74:76-87. doi: 10.1016/j.yjmcc.2014.04.020. Epub 2014 May 5. J Mol Cell Cardiol. 2014. PMID: 24805195 Free PMC article.
-
The K48-K63 Branched Ubiquitin Chain Regulates NF-κB Signaling.Mol Cell. 2016 Oct 20;64(2):251-266. doi: 10.1016/j.molcel.2016.09.014. Epub 2016 Oct 13. Mol Cell. 2016. PMID: 27746020
-
The role of post-translational modifications in cardiac hypertrophy.J Cell Mol Med. 2019 Jun;23(6):3795-3807. doi: 10.1111/jcmm.14330. Epub 2019 Apr 4. J Cell Mol Med. 2019. PMID: 30950211 Free PMC article. Review.
-
Atypical Ubiquitination and Parkinson's Disease.Int J Mol Sci. 2022 Mar 28;23(7):3705. doi: 10.3390/ijms23073705. Int J Mol Sci. 2022. PMID: 35409068 Free PMC article. Review.
Cited by
-
Ube2v1 Positively Regulates Protein Aggregation by Modulating Ubiquitin Proteasome System Performance Partially Through K63 Ubiquitination.Circ Res. 2020 Mar 27;126(7):907-922. doi: 10.1161/CIRCRESAHA.119.316444. Epub 2020 Feb 21. Circ Res. 2020. PMID: 32081062 Free PMC article.
-
Tripartite motif‑containing 14 may aggravate cardiac hypertrophy via the AKT signalling pathway in neonatal rat cardiomyocytes and transgenic mice.Mol Med Rep. 2023 Sep;28(3):173. doi: 10.3892/mmr.2023.13060. Epub 2023 Jul 28. Mol Med Rep. 2023. PMID: 37503784 Free PMC article.
-
Role and research progress of histone modification in cardiovascular diseases (Review).Exp Ther Med. 2025 May 13;30(1):132. doi: 10.3892/etm.2025.12882. eCollection 2025 Jul. Exp Ther Med. 2025. PMID: 40421232 Free PMC article. Review.
-
Cardiomyocyte-Enriched USP20 Ameliorates Pathological Cardiac Hypertrophy by Targeting STAT3 Deubiquitination.Adv Sci (Weinh). 2025 Jun;12(23):e2416478. doi: 10.1002/advs.202416478. Epub 2025 Apr 7. Adv Sci (Weinh). 2025. PMID: 40192103 Free PMC article.
-
Organ defects of the Usp7K444R mutant mouse strain indicate the essential role of K63-polyubiquitinated Usp7 in organ formation.Biomed J. 2023 Feb;46(1):122-133. doi: 10.1016/j.bj.2022.02.002. Epub 2022 Feb 18. Biomed J. 2023. PMID: 35183794 Free PMC article.
References
-
- Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochem Biophys Acta. 2004;1695:189‐207. - PubMed
-
- Teixeira LK, Reed SI. Ubiquitin ligases and cell cycle control. Annu Rev Biochem. 2013;82:387‐414. - PubMed
-
- Pickart CM. Back to the future with ubiquitin. Cell. 2004;116:181‐190. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

