Structural investigation of human S. aureus-targeting antibodies that bind wall teichoic acid
- PMID: 30102105
- PMCID: PMC6204806
- DOI: 10.1080/19420862.2018.1501252
Structural investigation of human S. aureus-targeting antibodies that bind wall teichoic acid
Abstract
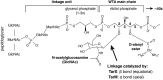
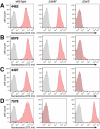
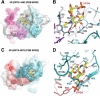
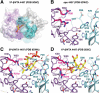
Infections caused by methicillin-resistant Staphylococcus aureus (MRSA) are a growing health threat worldwide. Efforts to identify novel antibodies that target S. aureus cell surface antigens are a promising direction in the development of antibiotics that can halt MRSA infection. We biochemically and structurally characterized three patient-derived MRSA-targeting antibodies that bind to wall teichoic acid (WTA), which is a polyanionic surface glycopolymer. In S. aureus, WTA exists in both α- and β-forms, based on the stereochemistry of attachment of a N-acetylglucosamine residue to the repeating phosphoribitol sugar unit. We identified a panel of antibodies cloned from human patients that specifically recognize the α or β form of WTA, and can bind with high affinity to pathogenic wild-type strains of S. aureus bacteria. To investigate how the β-WTA specific antibodies interact with their target epitope, we determined the X-ray crystal structures of the three β-WTA specific antibodies, 4462, 4497, and 6078 (Protein Data Bank IDs 6DWI, 6DWA, and 6DW2, respectively), bound to a synthetic WTA epitope. These structures reveal that all three of these antibodies, while utilizing distinct antibody complementarity-determining region sequences and conformations to interact with β-WTA, fulfill two recognition principles: binding to the β-GlcNAc pyranose core and triangulation of WTA phosphate residues with polar contacts. These studies reveal the molecular basis for targeting a unique S. aureus cell surface epitope and highlight the power of human patient-based antibody discovery techniques for finding novel pathogen-targeting therapeutics.
Keywords: Staphylococcus aureus; WTA; Wall Teichoic Acid; antibody structure; antibody-carbohydrate interactions; monoclonal antibodies.
Figures



Similar articles
-
Glycoepitopes of staphylococcal wall teichoic acid govern complement-mediated opsonophagocytosis via human serum antibody and mannose-binding lectin.J Biol Chem. 2013 Oct 25;288(43):30956-68. doi: 10.1074/jbc.M113.509893. Epub 2013 Sep 17. J Biol Chem. 2013. PMID: 24045948 Free PMC article. Clinical Trial.
-
The staphylococcal surface-glycopolymer wall teichoic acid (WTA) is crucial for complement activation and immunological defense against Staphylococcus aureus infection.Immunobiology. 2016 Oct;221(10):1091-101. doi: 10.1016/j.imbio.2016.06.003. Epub 2016 Jun 15. Immunobiology. 2016. PMID: 27424796 Review.
-
Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity.Nature. 2018 Nov;563(7733):705-709. doi: 10.1038/s41586-018-0730-x. Epub 2018 Nov 21. Nature. 2018. PMID: 30464342
-
Influence of Sodium Bicarbonate on Wall Teichoic Acid Synthesis and β-Lactam Sensitization in NaHCO3-Responsive and Nonresponsive Methicillin-Resistant Staphylococcus aureus.Microbiol Spectr. 2022 Dec 21;10(6):e0342222. doi: 10.1128/spectrum.03422-22. Epub 2022 Nov 15. Microbiol Spectr. 2022. PMID: 36377886 Free PMC article.
-
Pathways and roles of wall teichoic acid glycosylation in Staphylococcus aureus.Int J Med Microbiol. 2014 May;304(3-4):215-21. doi: 10.1016/j.ijmm.2013.10.009. Epub 2013 Nov 1. Int J Med Microbiol. 2014. PMID: 24365646 Review.
Cited by
-
Antibody Recognition of Different Staphylococcus aureus Wall Teichoic Acid Glycoforms.ACS Cent Sci. 2022 Oct 26;8(10):1383-1392. doi: 10.1021/acscentsci.2c00125. Epub 2022 Aug 17. ACS Cent Sci. 2022. PMID: 36313161 Free PMC article.
-
C1q binding to surface-bound IgG is stabilized by C1r2s2 proteases.Proc Natl Acad Sci U S A. 2021 Jun 29;118(26):e2102787118. doi: 10.1073/pnas.2102787118. Proc Natl Acad Sci U S A. 2021. PMID: 34155115 Free PMC article.
-
Anti-Hcp1 Monoclonal Antibody Is Protective against Burkholderia pseudomallei Infection via Recognizing Amino Acids at Asp95-Leu114.Pathogens. 2023 Dec 31;13(1):43. doi: 10.3390/pathogens13010043. Pathogens. 2023. PMID: 38251350 Free PMC article.
-
The Orphan Immune Receptor LILRB3 Modulates Fc Receptor-Mediated Functions of Neutrophils.J Immunol. 2020 Feb 15;204(4):954-966. doi: 10.4049/jimmunol.1900852. Epub 2020 Jan 8. J Immunol. 2020. PMID: 31915259 Free PMC article.
-
(Automated) Synthesis of Well-defined Staphylococcus Aureus Wall Teichoic Acid Fragments.Chemistry. 2021 Jul 16;27(40):10461-10469. doi: 10.1002/chem.202101242. Epub 2021 Jun 9. Chemistry. 2021. PMID: 33991006 Free PMC article.
References
-
- Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, RN Jones, Beach M. SENTRY Partcipants Group . Survey of infections due to staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997-1999. Clin Infect Dis. 2001;32(Suppl 2):S114–32. doi: 10.1086/318467. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
