Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo
- PMID: 30102295
- PMCID: PMC6126939
- DOI: 10.1038/nbt.4195
Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo
Abstract
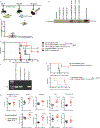
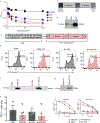
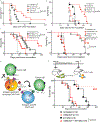
The efficacy of chimeric antigen receptor (CAR) T cell therapy against poorly responding tumors can be enhanced by administering the cells in combination with immune checkpoint blockade inhibitors. Alternatively, the CAR construct has been engineered to coexpress factors that boost CAR-T cell function in the tumor microenvironment. We modified CAR-T cells to secrete PD-1-blocking single-chain variable fragments (scFv). These scFv-secreting CAR-T cells acted in both a paracrine and autocrine manner to improve the anti-tumor activity of CAR-T cells and bystander tumor-specific T cells in clinically relevant syngeneic and xenogeneic mouse models of PD-L1+ hematologic and solid tumors. The efficacy was similar to or better than that achieved by combination therapy with CAR-T cells and a checkpoint inhibitor. This approach may improve safety, as the secreted scFvs remained localized to the tumor, protecting CAR-T cells from PD-1 inhibition, which could potentially avoid toxicities associated with systemic checkpoint inhibition.
Conflict of interest statement
Figures



References
Methods-only References
-
- Shevchenko A, Tomas H, Havlis J, Olsen JV & Mann M In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1, 2856–2860 (2006). - PubMed
-
- Rappsilber J, Mann M & Ishihama Y Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc 2, 1896–1906 (2007). - PubMed
-
- Lee AY et al. Measurement of fractional synthetic rates of multiple protein analytes by triple quadrupole mass spectrometry. Clin Chem 58, 619–627 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

