In vivo genome-wide binding interactions of mouse and human constitutive androstane receptors reveal novel gene targets
- PMID: 30102401
- PMCID: PMC6144799
- DOI: 10.1093/nar/gky692
In vivo genome-wide binding interactions of mouse and human constitutive androstane receptors reveal novel gene targets
Abstract
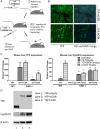
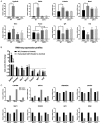
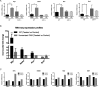
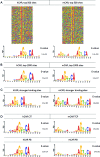
The constitutive androstane receptor (CAR; NR1I3) is a nuclear receptor orchestrating complex roles in cell and systems biology. Species differences in CAR's effector pathways remain poorly understood, including its role in regulating liver tumor promotion. We developed transgenic mouse models to assess genome-wide binding of mouse and human CAR, following receptor activation in liver with direct ligands and with phenobarbital, an indirect CAR activator. Genomic interaction profiles were integrated with transcriptional and biological pathway analyses. Newly identified CAR target genes included Gdf15 and Foxo3, important regulators of the carcinogenic process. Approximately 1000 genes exhibited differential binding interactions between mouse and human CAR, including the proto-oncogenes, Myc and Ikbke, which demonstrated preferential binding by mouse CAR as well as mouse CAR-selective transcriptional enhancement. The ChIP-exo analyses also identified distinct binding motifs for the respective mouse and human receptors. Together, the results provide new insights into the important roles that CAR contributes as a key modulator of numerous signaling pathways in mammalian organisms, presenting a genomic context that specifies species variation in biological processes under CAR's control, including liver cell proliferation and tumor promotion.
Figures




Similar articles
-
Bioinformatic analysis of microRNA networks following the activation of the constitutive androstane receptor (CAR) in mouse liver.Biochim Biophys Acta. 2016 Sep;1859(9):1228-1237. doi: 10.1016/j.bbagrm.2016.04.002. Epub 2016 Apr 11. Biochim Biophys Acta. 2016. PMID: 27080131 Free PMC article.
-
Stilbene compound trans-3,4,5,4´-tetramethoxystilbene, a potential anticancer drug, regulates constitutive androstane receptor (Car) target genes, but does not possess proliferative activity in mouse liver.Toxicol Lett. 2019 Oct 1;313:1-10. doi: 10.1016/j.toxlet.2019.05.024. Epub 2019 Jun 4. Toxicol Lett. 2019. PMID: 31170421
-
Multi-species analyses of direct activators of the constitutive androstane receptor.Toxicol Sci. 2011 Oct;123(2):550-62. doi: 10.1093/toxsci/kfr191. Epub 2011 Jul 21. Toxicol Sci. 2011. PMID: 21778469 Free PMC article.
-
Signaling control of the constitutive androstane receptor (CAR).Protein Cell. 2014 Feb;5(2):113-23. doi: 10.1007/s13238-013-0013-0. Epub 2014 Jan 29. Protein Cell. 2014. PMID: 24474196 Free PMC article. Review.
-
Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: A case study with phenobarbital as a model constitutive androstane receptor (CAR) activator.Crit Rev Toxicol. 2014 Jan;44(1):64-82. doi: 10.3109/10408444.2013.835786. Epub 2013 Nov 4. Crit Rev Toxicol. 2014. PMID: 24180433 Free PMC article. Review.
Cited by
-
Pharmacological Activation of PXR and CAR Downregulates Distinct Bile Acid-Metabolizing Intestinal Bacteria and Alters Bile Acid Homeostasis.Toxicol Sci. 2019 Mar 1;168(1):40-60. doi: 10.1093/toxsci/kfy271. Toxicol Sci. 2019. PMID: 30407581 Free PMC article.
-
CAR directs T cell adaptation to bile acids in the small intestine.Nature. 2021 May;593(7857):147-151. doi: 10.1038/s41586-021-03421-6. Epub 2021 Apr 7. Nature. 2021. PMID: 33828301 Free PMC article.
-
Impact of Neonatal Activation of Nuclear Receptor CAR (Nr1i3) on Cyp2 Gene Expression in Adult Mouse Liver.Toxicol Sci. 2022 May 26;187(2):298-310. doi: 10.1093/toxsci/kfac032. Toxicol Sci. 2022. PMID: 35285501 Free PMC article.
-
Genome-wide expression profiling reveals increased stability and mitochondrial energy metabolism of the human liver cell line HepaRG-CAR.Cytotechnology. 2020 Jun;72(3):377-395. doi: 10.1007/s10616-020-00384-w. Epub 2020 Mar 4. Cytotechnology. 2020. PMID: 32130581 Free PMC article.
-
Pharmacological activation of constitutive androstane receptor induces female-specific modulation of hepatic metabolism.JHEP Rep. 2023 Oct 13;6(1):100930. doi: 10.1016/j.jhepr.2023.100930. eCollection 2024 Jan. JHEP Rep. 2023. PMID: 38149074 Free PMC article.
References
-
- Steinmetz A.C., Renaud J.P., Moras D.. Binding of ligands and activation of transcription by nuclear receptors. Annu. Rev. Biophys. Biomol. Struct. 2001; 30:329–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

