BMP-2 induces human mononuclear cell chemotaxis and adhesion and modulates monocyte-to-macrophage differentiation
- PMID: 30102472
- PMCID: PMC6201342
- DOI: 10.1111/jcmm.13814
BMP-2 induces human mononuclear cell chemotaxis and adhesion and modulates monocyte-to-macrophage differentiation
Abstract
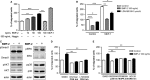
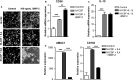
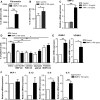
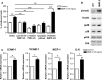
Type 2 diabetes mellitus (T2DM) is a cardiovascular risk factor which leads to atherosclerosis, an inflammatory disease characterized by the infiltration of mononuclear cells in the vessel. Bone morphogenetic protein (BMP)-2 is a cytokine which has been recently shown to be elevated in atherosclerosis and T2DM and to contribute to vascular inflammation. However, the role of BMP-2 in the regulation of mononuclear cell function remains to be established. Herein, we demonstrate that BMP-2 induced human monocyte chemotaxis via phosphoinositide 3 kinase and mitogen-activated protein kinases. Inhibition of endogenous BMP-2 signalling, by Noggin or a BMP receptor inhibitor, interfered with monocyte migration. Although BMP-2 expression was increased in monocytes from T2DM patients, it could still stimulate their migration. Furthermore, BMP-2 interfered with their differentiation into M2 macrophages. Finally, BMP-2 both induced the adhesion of monocytes to fibronectin and endothelial cells (ECs), and promoted the adhesive properties of ECs, by increasing expression of adhesion and pro-inflammatory molecules. Our data demonstrate that BMP-2 could exert its pro-inflammatory effects by inducing monocyte migration and adhesiveness to ECs and by interfering with the monocyte differentiation into M2 macrophages. Our findings provide novel insights into the mechanisms by which BMP-2 may contribute to the development of atherosclerosis.
Keywords: BMP; adhesion; atherosclerosis; chemotaxis; diabetes; endothelial cells; monocytes.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

References
-
- Vattikuti R, Towler DA. Osteogenic regulation of vascular calcification: an early perspective. Am J Physiol Endocrinol Metab. 2004;286:E686‐E696. - PubMed
-
- Pardali E, Waltenberger J. Monocyte function and trafficking in cardiovascular disease. Thromb Haemost. 2012;108:804‐811. - PubMed
-
- Tchaikovski V, Olieslagers S, Bohmer FD, Waltenberger J. Diabetes mellitus activates signal transduction pathways resulting in vascular endothelial growth factor resistance of human monocytes. Circulation. 2009;120:150‐159. - PubMed
-
- Cai J, Pardali E, Sanchez‐Duffhues G, ten Dijke P. Bmp signaling in vascular diseases. FEBS Lett. 2012;586:1993‐2002. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

