Chemical Structure and Composition of Major Glycans Covalently Linked to Therapeutic Monoclonal Antibodies by Middle-Down Nuclear Magnetic Resonance
- PMID: 30102512
- PMCID: PMC7040853
- DOI: 10.1021/acs.analchem.8b02637
Chemical Structure and Composition of Major Glycans Covalently Linked to Therapeutic Monoclonal Antibodies by Middle-Down Nuclear Magnetic Resonance
Abstract
Glycosylation of monoclonal antibodies (mAbs) is a critical quality attribute that can impact mAb drug efficacy and safety. The mAb glycans are inherently heterogeneous in chemical structure and composition of monosaccharides. The established fluorescence or mass-spectrometry (MS) detection methods for glycosylation evaluation may require multiple steps of glycan cleavage or extensive digestion of the mAb, chemical labeling of the glycans, column separation and report the chemical identity of glycans indirectly through retention time and molecular weight values. In demonstrating chemical structure similarity and comparability among mAb drugs, orthogonal analytical methods for measuring glycan chemistry are needed to ensure the quality of drug products. Here, a "middle-down" NMR method is developed as a proof-of-concept approach to measure the domain-specific glycosylation of marketed mAb drugs without cleavage of the glycan moieties. Complete glycan 1H/13C chemical shift assignments were obtained at 13C natural abundance from commercial standard glycans that allowed unambiguous determination of the chemical structure, glycosidic linkage position, and anomeric configuration of each monosaccharide in the major N-glycan scaffolds found in mAb molecules. The analysis of glycan anomeric peaks in two-dimensional (2D) 1H-13C NMR spectra yielded metrics for clinically important mAb quality attributes (i.e., galactosylation (Gal%) and fucosylation (Fuc%)), consistent with literature results using a standard glycan-mapping method. Therefore, the middle-down NMR method provided a facile orthogonal measurement for mAb glycosylation characterization with improved chemical information content on glycan structure determination and quantification, compared to standard approaches.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
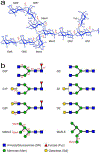

Similar articles
-
Assessment of monoclonal antibody glycosylation: a comparative study using HRMS, NMR, and HILIC-FLD.Anal Bioanal Chem. 2024 May;416(13):3127-3137. doi: 10.1007/s00216-024-05261-5. Epub 2024 Apr 6. Anal Bioanal Chem. 2024. PMID: 38580890 Free PMC article.
-
Minor N-Glycan Mapping of Monoclonal Antibody Therapeutics Using Middle-Down NMR Spectroscopy.Mol Pharm. 2021 Jan 4;18(1):441-450. doi: 10.1021/acs.molpharmaceut.0c01083. Epub 2020 Dec 11. Mol Pharm. 2021. PMID: 33305950
-
Comparison of orthogonal chromatographic and lectin-affinity microarray methods for glycan profiling of a therapeutic monoclonal antibody.J Chromatogr B Analyt Technol Biomed Life Sci. 2015 Aug 1;997:162-78. doi: 10.1016/j.jchromb.2015.05.035. Epub 2015 Jun 9. J Chromatogr B Analyt Technol Biomed Life Sci. 2015. PMID: 26114652
-
Antibody glycosylation and its impact on the pharmacokinetics and pharmacodynamics of monoclonal antibodies and Fc-fusion proteins.J Pharm Sci. 2015 Jun;104(6):1866-1884. doi: 10.1002/jps.24444. Epub 2015 Apr 14. J Pharm Sci. 2015. PMID: 25872915 Review.
-
Analysis of glycoforms on the glycosylation site and the glycans in monoclonal antibody biopharmaceuticals.J Sep Sci. 2012 Feb;35(3):341-50. doi: 10.1002/jssc.201100684. Epub 2011 Dec 27. J Sep Sci. 2012. PMID: 22213703 Review.
Cited by
-
Assessment of monoclonal antibody glycosylation: a comparative study using HRMS, NMR, and HILIC-FLD.Anal Bioanal Chem. 2024 May;416(13):3127-3137. doi: 10.1007/s00216-024-05261-5. Epub 2024 Apr 6. Anal Bioanal Chem. 2024. PMID: 38580890 Free PMC article.
-
The impact of glycosylation on the structure, function, and interactions of CD14.Glycobiology. 2024 Apr 1;34(3):cwae002. doi: 10.1093/glycob/cwae002. Glycobiology. 2024. PMID: 38227775 Free PMC article.
-
Unambiguous identification of α-Gal epitopes in intact monoclonal antibodies by NMR spectroscopy.MAbs. 2022 Jan-Dec;14(1):2132977. doi: 10.1080/19420862.2022.2132977. MAbs. 2022. PMID: 36239533 Free PMC article.
-
Unambiguous Identification of Glucose-Induced Glycation in mAbs and other Proteins by NMR Spectroscopy.Pharm Res. 2023 Jun;40(6):1341-1353. doi: 10.1007/s11095-022-03454-0. Epub 2022 Dec 13. Pharm Res. 2023. PMID: 36510116 Free PMC article.
-
Glycoprofile Analysis of an Intact Glycoprotein As Inferred by NMR Spectroscopy.ACS Cent Sci. 2019 Sep 25;5(9):1554-1561. doi: 10.1021/acscentsci.9b00540. Epub 2019 Jul 24. ACS Cent Sci. 2019. PMID: 31572782 Free PMC article.
References
-
- Cai HH MOJ Immunol. 2017, 5 (1), 00145.
-
- Liu LM J. Pharm. Sci 2015, 104, 1866–1884. - PubMed
-
- Hmiel LK; Brorson KA; Boyne MT Anal. Bioanal. Chem 2015, 407, 79–94. - PubMed
-
- Hossler P; Khattak SF; Li ZJ Glycobiology 2009, 19, 936–949. - PubMed
-
- Zhang PQ; Woen S; Wang TH; Liau B; Zhao S; Chen C; Yang YS; Song ZW; Wormald MR; Yu CF; Rudd PM Drug Discovery Today 2016, 21, 740–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

