Chemical Structure and Composition of Major Glycans Covalently Linked to Therapeutic Monoclonal Antibodies by Middle-Down Nuclear Magnetic Resonance
- PMID: 30102512
- PMCID: PMC7040853
- DOI: 10.1021/acs.analchem.8b02637
Chemical Structure and Composition of Major Glycans Covalently Linked to Therapeutic Monoclonal Antibodies by Middle-Down Nuclear Magnetic Resonance
Abstract
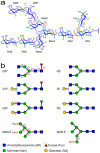
Glycosylation of monoclonal antibodies (mAbs) is a critical quality attribute that can impact mAb drug efficacy and safety. The mAb glycans are inherently heterogeneous in chemical structure and composition of monosaccharides. The established fluorescence or mass-spectrometry (MS) detection methods for glycosylation evaluation may require multiple steps of glycan cleavage or extensive digestion of the mAb, chemical labeling of the glycans, column separation and report the chemical identity of glycans indirectly through retention time and molecular weight values. In demonstrating chemical structure similarity and comparability among mAb drugs, orthogonal analytical methods for measuring glycan chemistry are needed to ensure the quality of drug products. Here, a "middle-down" NMR method is developed as a proof-of-concept approach to measure the domain-specific glycosylation of marketed mAb drugs without cleavage of the glycan moieties. Complete glycan 1H/13C chemical shift assignments were obtained at 13C natural abundance from commercial standard glycans that allowed unambiguous determination of the chemical structure, glycosidic linkage position, and anomeric configuration of each monosaccharide in the major N-glycan scaffolds found in mAb molecules. The analysis of glycan anomeric peaks in two-dimensional (2D) 1H-13C NMR spectra yielded metrics for clinically important mAb quality attributes (i.e., galactosylation (Gal%) and fucosylation (Fuc%)), consistent with literature results using a standard glycan-mapping method. Therefore, the middle-down NMR method provided a facile orthogonal measurement for mAb glycosylation characterization with improved chemical information content on glycan structure determination and quantification, compared to standard approaches.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Cai HH MOJ Immunol. 2017, 5 (1), 00145.
-
- Liu LM J. Pharm. Sci 2015, 104, 1866–1884. - PubMed
-
- Hmiel LK; Brorson KA; Boyne MT Anal. Bioanal. Chem 2015, 407, 79–94. - PubMed
-
- Hossler P; Khattak SF; Li ZJ Glycobiology 2009, 19, 936–949. - PubMed
-
- Zhang PQ; Woen S; Wang TH; Liau B; Zhao S; Chen C; Yang YS; Song ZW; Wormald MR; Yu CF; Rudd PM Drug Discovery Today 2016, 21, 740–765. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

