Macrophage-derived LTB4 promotes abscess formation and clearance of Staphylococcus aureus skin infection in mice
- PMID: 30102746
- PMCID: PMC6107286
- DOI: 10.1371/journal.ppat.1007244
Macrophage-derived LTB4 promotes abscess formation and clearance of Staphylococcus aureus skin infection in mice
Abstract
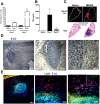
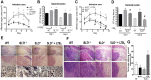
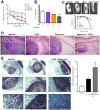
The early events that shape the innate immune response to restrain pathogens during skin infections remain elusive. Methicillin-resistant Staphylococcus aureus (MRSA) infection engages phagocyte chemotaxis, abscess formation, and microbial clearance. Upon infection, neutrophils and monocytes find a gradient of chemoattractants that influence both phagocyte direction and microbial clearance. The bioactive lipid leukotriene B4 (LTB4) is quickly (seconds to minutes) produced by 5-lipoxygenase (5-LO) and signals through the G protein-coupled receptors LTB4R1 (BLT1) or BLT2 in phagocytes and structural cells. Although it is known that LTB4 enhances antimicrobial effector functions in vitro, whether prompt LTB4 production is required for bacterial clearance and development of an inflammatory milieu necessary for abscess formation to restrain pathogen dissemination is unknown. We found that LTB4 is produced in areas near the abscess and BLT1 deficient mice are unable to form an abscess, elicit neutrophil chemotaxis, generation of neutrophil and monocyte chemokines, as well as reactive oxygen species-dependent bacterial clearance. We also found that an ointment containing LTB4 synergizes with antibiotics to eliminate MRSA potently. Here, we uncovered a heretofore unknown role of macrophage-derived LTB4 in orchestrating the chemoattractant gradient required for abscess formation, while amplifying antimicrobial effector functions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Comment in
-
Fatal chemoattraction.Nat Chem Biol. 2018 Oct;14(10):903. doi: 10.1038/s41589-018-0140-2. Nat Chem Biol. 2018. PMID: 30224689 No abstract available.
References
-
- Trividic M, Gauthier ML, Sparsa A, Ploy MC, Mounier M, Boulinguez S, et al. [Methi-resistant Staphylococcus aureus in dermatological practice: origin, risk factors and outcome]. Ann Dermatol Venereol. 2002;129(1 Pt 1):27–9. Epub 2002/04/09. . - PubMed
-
- Vayalumkal JV, Whittingham H, Vanderkooi O, Stewart TE, Low DE, Mulvey M, et al. Necrotizing pneumonia and septic shock: suspecting CA-MRSA in patients presenting to Canadian emergency departments. CJEM. 2007;9(4):300–3. . - PubMed
-
- Gessler P, Pretre R, Hohl V, Rousson V, Fischer J, Dahinden C. CXC-chemokine stimulation of neutrophils correlates with plasma levels of myeloperoxidase and lactoferrin and contributes to clinical outcome after pediatric cardiac surgery. Shock. 2004;22(6):513–20. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

