Macrophage-Specific Hypoxia-Inducible Factor-1α Contributes to Impaired Autophagic Flux in Nonalcoholic Steatohepatitis
- PMID: 30102772
- PMCID: PMC6351177
- DOI: 10.1002/hep.30215
Macrophage-Specific Hypoxia-Inducible Factor-1α Contributes to Impaired Autophagic Flux in Nonalcoholic Steatohepatitis
Abstract
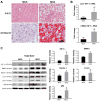
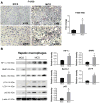
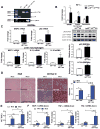
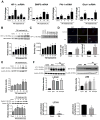
Inflammatory cell activation drives diverse cellular programming during hepatic diseases. Hypoxia-inducible factors (HIFs) have recently been identified as important regulators of immunity and inflammation. In nonalcoholic steatohepatitis (NASH), HIF-1α is upregulated in hepatocytes, where it induces steatosis; however, the role of HIF-1α in macrophages under metabolic stress has not been explored. In this study, we found increased HIF-1α levels in hepatic macrophages in methionine-choline-deficient (MCD) diet-fed mice and in macrophages of patients with NASH compared with controls. The HIF-1α increase was concomitant with elevated levels of autophagy markers BNIP3, Beclin-1, LC3-II, and p62 in both mouse and human macrophages. LysMCre HIFdPA fl/fl mice, which have HIF-1α levels stabilized in macrophages, showed higher steatosis and liver inflammation compared with HIFdPA fl/fl mice on MCD diet. In vitro and ex vivo experiments reveal that saturated fatty acid, palmitic acid (PA), both induces HIF-1α and impairs autophagic flux in macrophages. Using small interfering RNA-mediated knock-down and overexpression of HIF-1α in macrophages, we demonstrated that PA impairs autophagy via HIF-1α. We found that HIF-1α mediates NF-κB activation and MCP-1 production and that HIF-1α-mediated impairment of macrophage autophagy increases IL-1β production, contributing to MCD diet-induced NASH. Conclusion: Palmitic acid impairs autophagy via HIF-1α activation in macrophages. HIF-1α and impaired autophagy are present in NASH in vivo in mouse macrophages and in human blood monocytes. We identified that HIF-1α activation and decreased autophagic flux stimulate inflammation in macrophages through upregulation of NF-κB activation. These results suggest that macrophage activation in NASH involves a complex interplay between HIF-1α and autophagy as these pathways promote proinflammatory overactivation in MCD diet-induced NASH.
© 2018 by the American Association for the Study of Liver Diseases.
Figures




References
-
- Chitturi S, Wong VW, Farrell G. Nonalcoholic fatty liver in Asia: Firmly entrenched and rapidly gaining ground. J Gastroenterol Hepatol. 2011;26(Suppl 1):163–172. - PubMed
-
- Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des. 2010;16:1941–1951. - PubMed
-
- Musso G, Cassader M, Olivetti C, Rosina F, Carbone G, Gambino R. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

