Effect of 1- and 2-Month High-Dose Alpha-Linolenic Acid Treatment on 13 C-Labeled Alpha-Linolenic Acid Incorporation and Conversion in Healthy Subjects
- PMID: 30102841
- PMCID: PMC6646899
- DOI: 10.1002/mnfr.201800271
Effect of 1- and 2-Month High-Dose Alpha-Linolenic Acid Treatment on 13 C-Labeled Alpha-Linolenic Acid Incorporation and Conversion in Healthy Subjects
Abstract
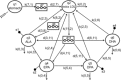
Scope: The study aims at identifying 1) the most sensitive compartment among plasma phospholipids, erythrocytes, and LDL for studying alpha-linolenic acid (ALA) conversion, and 2) whether ALA incorporation and conversion is saturable after administration of 13 C-labeled ALA-rich linseed oil (LO). The effect of a daily intake of 7 g nonlabeled LO (>43% w/w ALA) for 1 month after bolus administration of 7 g 13 C-labeled LO on day 1, and for 2 months after bolus administration of 7 g 13 C-labeled LO on day 1 and day 29 on 13 C-ALA incorporation and conversion into its higher homologs is investigated in healthy volunteers.
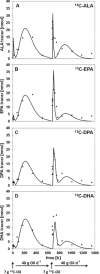
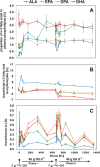
Methods and results: Incorporation and conversion of LO-derived 13 C-labeled ALA is quantified by applying compartmental modeling. After bolus administration, a fractional conversion of approximately 30% from 13 C-ALA to 13 C-DHA is calculated as reflected by the LDL compartment. Treatment with LO for 8 weeks induces a mean reduction of 13 C-ALA conversion to 13 C-DHA by 48% as reflected by the LDL compartment, and a mean reduction of the 13 C-ALA incorporation into LDL by 46%.
Conclusion: A 2-month dietary intake of a high dose of LO is sufficient to reach saturation of ALA incorporation into LDL particles, which are responsible for ALA distribution in the body.
Keywords: ALA conversion; LDL; compartment model; linseed oil; omega-3 fatty acids.
© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Short-term supplementation of low-dose gamma-linolenic acid (GLA), alpha-linolenic acid (ALA), or GLA plus ALA does not augment LCP omega 3 status of Dutch vegans to an appreciable extent.Prostaglandins Leukot Essent Fatty Acids. 2000 Nov;63(5):287-92. doi: 10.1054/plef.2000.0216. Prostaglandins Leukot Essent Fatty Acids. 2000. PMID: 11090255
-
Influence of three rapeseed oil-rich diets, fortified with alpha-linolenic acid, eicosapentaenoic acid or docosahexaenoic acid on the composition and oxidizability of low-density lipoproteins: results of a controlled study in healthy volunteers.Eur J Clin Nutr. 2007 Mar;61(3):314-25. doi: 10.1038/sj.ejcn.1602523. Epub 2006 Sep 13. Eur J Clin Nutr. 2007. PMID: 16969378 Clinical Trial.
-
Effects of the flavonol quercetin and α-linolenic acid on n-3 PUFA status in metabolically healthy men and women: a randomised, double-blinded, placebo-controlled, crossover trial.Br J Nutr. 2017 Mar;117(5):698-711. doi: 10.1017/S0007114517000241. Epub 2017 Apr 3. Br J Nutr. 2017. PMID: 28366181 Clinical Trial.
-
Metabolism and functional effects of plant-derived omega-3 fatty acids in humans.Prog Lipid Res. 2016 Oct;64:30-56. doi: 10.1016/j.plipres.2016.07.002. Epub 2016 Aug 3. Prog Lipid Res. 2016. PMID: 27496755 Review.
-
alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans.Prostaglandins Leukot Essent Fatty Acids. 2009 Feb-Mar;80(2-3):85-91. doi: 10.1016/j.plefa.2009.01.004. Epub 2009 Mar 9. Prostaglandins Leukot Essent Fatty Acids. 2009. PMID: 19269799 Review.
Cited by
-
Omega-3 nutraceuticals, climate change and threats to the environment: The cases of Antarctic krill and Calanus finmarchicus.Ambio. 2021 Jun;50(6):1184-1199. doi: 10.1007/s13280-020-01472-z. Epub 2021 Jan 27. Ambio. 2021. PMID: 33502683 Free PMC article.
References
-
- Burr M. L., Fehily A. M., Gilbert J. F., Rogers S., Holliday R. M., Sweetnam P. M., Elwood P. C., Deadman N. M., Lancet 1989, 2, 757. - PubMed
-
- Sarwar N., Danesh J., Eiriksdottir G., Sigurdsson G., Wareham N., Bingham S., Boekholdt S. M., Khaw K. T., Gudnason V., Circulation 2007, 115, 450. - PubMed
-
- Abeywardena M. Y., Patten G. S., Endocr. Metab. Immune Disord. Drug Targets 2011, 11, 232. - PubMed
-
- Calder P. C., Mol. Nutr. Food Res. 2008, 52, 885. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

