Cardiac-specific knockout of Lmod2 results in a severe reduction in myofilament force production and rapid cardiac failure
- PMID: 30102883
- PMCID: PMC6324932
- DOI: 10.1016/j.yjmcc.2018.08.009
Cardiac-specific knockout of Lmod2 results in a severe reduction in myofilament force production and rapid cardiac failure
Abstract
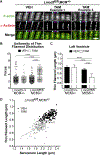
Leiomodin-2 (Lmod2) is a striated muscle-specific actin binding protein that is implicated in assembly of thin filaments. The necessity of Lmod2 in the adult mouse and role it plays in the mechanics of contraction are unknown. To answer these questions, we generated cardiac-specific conditional Lmod2 knockout mice (cKO). These mice die within a week of induction of the knockout with severe left ventricular systolic dysfunction and little change in cardiac morphology. Cardiac trabeculae isolated from cKO mice have a significant decrease in maximum force production and a blunting of myofilament length-dependent activation. Thin filaments are non-uniform and substantially reduced in length in cKO hearts, affecting the functional overlap of the thick and thin filaments. Remarkably, we also found that Lmod2 levels are directly linked to thin filament length and cardiac function in vivo, with a low amount (<20%) of Lmod2 necessary to maintain cardiac function. Thus, Lmod2 plays an essential role in maintaining proper cardiac thin filament length in adult mice, which in turn is necessary for proper generation of contractile force. Dysregulation of thin filament length in the absence of Lmod2 contributes to heart failure.
Keywords: Actin-thin filaments; Cardiomyopathy; Sarcomere.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
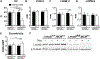



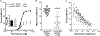

References
-
- Winter JM, Joureau B, Lee EJ, Kiss B, Yuen M, Gupta VA, Pappas CT, Gregorio CC, Stienen GJ, Edvardson S, Wallgren-Pettersson C, Lehtokari VL, Pelin K, Malfatti E, Romero NB, Engelen BG, Voermans NC, Donkervoort S, Bonnemann CG, Clarke NF, Beggs AH, Granzier H, Ottenheijm CA, Mutation-specific effects on thin filament length in thin filament myopathy, Ann Neurol 79(6) (2016) 959–69. - PMC - PubMed
-
- Yuen M, Sandaradura SA, Dowling JJ, Kostyukova AS, Moroz N, Quinlan KG, Lehtokari VL, Ravenscroft G, Todd EJ, Ceyhan-Birsoy O, Gokhin DS, Maluenda J, Lek M, Nolent F, Pappas CT, Novak SM, D’Amico A, Malfatti E, Thomas BP, Gabriel SB, Gupta N, Daly MJ, Ilkovski B, Houweling PJ, Davidson AE, Swanson LC, Brownstein CA, Gupta VA, Medne L, Shannon P, Martin N, Bick DP, Flisberg A, Holmberg E, Van den Bergh P, Lapunzina P, Waddell LB, Sloboda DD, Bertini E, Chitayat D, Telfer WR, Laquerriere A, Gregorio CC, Ottenheijm CA, Bonnemann CG, Pelin K, Beggs AH, Hayashi YK, Romero NB, Laing NG, Nishino I, Wallgren-Pettersson C, Melki J, Fowler VM, MacArthur DG, North KN, Clarke NF, Leiomodin-3 dysfunction results in thin filament disorganization and nemaline myopathy, J Clin Invest 124(11) (2014) 4693–708. - PMC - PubMed
-
- Conley CA, Fritz-Six KL, Almenar-Queralt A, Fowler VM, Leiomodins: larger members of the tropomodulin (Tmod) gene family, Genomics 73(2) (2001) 127–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

