Validation of Babesia proteasome as a drug target
- PMID: 30103207
- PMCID: PMC6092455
- DOI: 10.1016/j.ijpddr.2018.08.001
Validation of Babesia proteasome as a drug target
Abstract
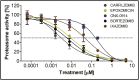
Babesiosis is a tick-transmitted zoonosis caused by apicomplexan parasites of the genus Babesia. Treatment of this emerging malaria-related disease has relied on antimalarial drugs and antibiotics. The proteasome of Plasmodium, the causative agent of malaria, has recently been validated as a target for anti-malarial drug development and therefore, in this study, we investigated the effect of epoxyketone (carfilzomib, ONX-0914 and epoxomicin) and boronic acid (bortezomib and ixazomib) proteasome inhibitors on the growth and survival of Babesia. Testing the compounds against Babesia divergens ex vivo revealed suppressive effects on parasite growth with activity that was higher than the cytotoxic effects on a non-transformed mouse macrophage cell line. Furthermore, we showed that the most-effective compound, carfilzomib, significantly reduces parasite multiplication in a Babesia microti infected mouse model without noticeable adverse effects. In addition, treatment with carfilzomib lead to an ex vivo and in vivo decrease in proteasome activity and accumulation of polyubiquitinated proteins compared to untreated control. Overall, our results demonstrate that the Babesia proteasome is a valid target for drug development and warrants the design of potent and selective B. divergens proteasome inhibitors for the treatment of babesiosis.
Keywords: Babesia; Carfilzomib; Cytotoxicity; Epoxyketone; Proteasome.
Copyright © 2018. Published by Elsevier Ltd.
Figures








Similar articles
-
Evaluating Antimalarial Proteasome Inhibitors for Efficacy in Babesia Blood Stage Cultures.ACS Omega. 2024 Oct 28;9(45):44989-44999. doi: 10.1021/acsomega.4c04564. eCollection 2024 Nov 12. ACS Omega. 2024. PMID: 39554424 Free PMC article.
-
Effect of protease inhibitors on the intraerythrocytic development of Babesia microti and Babesia duncani, the causative agents of human babesiosis.J Eukaryot Microbiol. 2025 Mar-Apr;72(2):e13064. doi: 10.1111/jeu.13064. Epub 2024 Nov 18. J Eukaryot Microbiol. 2025. PMID: 39556081 Free PMC article.
-
Ellagic acid microspheres restrict the growth of Babesia and Theileria in vitro and Babesia microti in vivo.Parasit Vectors. 2019 May 28;12(1):269. doi: 10.1186/s13071-019-3520-x. Parasit Vectors. 2019. PMID: 31138282 Free PMC article.
-
[Babesiosis, a little known zoonosis].Epidemiol Mikrobiol Imunol. 2007 Nov;56(4):176-80. Epidemiol Mikrobiol Imunol. 2007. PMID: 18072299 Review. Czech.
-
Unravelling the cellular and molecular pathogenesis of bovine babesiosis: is the sky the limit?Int J Parasitol. 2019 Feb;49(2):183-197. doi: 10.1016/j.ijpara.2018.11.002. Epub 2019 Jan 26. Int J Parasitol. 2019. PMID: 30690089 Free PMC article. Review.
Cited by
-
p300/CBP inhibitor A-485 alleviates acute liver injury by regulating macrophage activation and polarization.Theranostics. 2019 Oct 22;9(26):8344-8361. doi: 10.7150/thno.30707. eCollection 2019. Theranostics. 2019. PMID: 31754401 Free PMC article.
-
In Silico Survey and Characterization of Babesia microti Functional and Non-Functional Proteases.Pathogens. 2021 Nov 10;10(11):1457. doi: 10.3390/pathogens10111457. Pathogens. 2021. PMID: 34832610 Free PMC article.
-
Distinct substrate specificities of the three catalytic subunits of the Trichomonas vaginalis proteasome.Protein Sci. 2024 Dec;33(12):e5225. doi: 10.1002/pro.5225. Protein Sci. 2024. PMID: 39589076
-
Chelation of Mitochondrial Iron as an Antiparasitic Strategy.ACS Infect Dis. 2024 Feb 9;10(2):676-687. doi: 10.1021/acsinfecdis.3c00529. Epub 2024 Jan 30. ACS Infect Dis. 2024. PMID: 38287902 Free PMC article.
-
Evaluating Antimalarial Proteasome Inhibitors for Efficacy in Babesia Blood Stage Cultures.ACS Omega. 2024 Oct 28;9(45):44989-44999. doi: 10.1021/acsomega.4c04564. eCollection 2024 Nov 12. ACS Omega. 2024. PMID: 39554424 Free PMC article.
References
-
- Aboulaila M., Nakamura K., Govind Y., Yokoyama N., Igarashi I. Evaluation of the in vitro growth-inhibitory effect of epoxomicin on Babesia parasites. Vet. Parasitol. 2010;167:19–27. - PubMed
-
- Adams J. The proteasome: a suitable antineoplastic target. Nat. Rev. Canc. 2004;4:349–360. - PubMed
-
- Arastu-Kapur S., Anderl J.L., Kraus M., Parlati F., Shenk K.D., Lee S.J., Muchamuel T., Bennett M.K., Driessen C., Ball A.J., Kirk C.J. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin. Canc. Res. 2011;17:2734–2743. - PubMed
-
- Arisue N., Hashimoto T. Phylogeny and evolution of apicoplasts and apicomplexan parasites. Parasitol. Int. 2015;64:254–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

