Murine Bone Marrow Niches from Hematopoietic Stem Cells to B Cells
- PMID: 30103411
- PMCID: PMC6121419
- DOI: 10.3390/ijms19082353
Murine Bone Marrow Niches from Hematopoietic Stem Cells to B Cells
Abstract
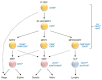
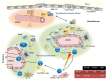
After birth, the development of hematopoietic cells occurs in the bone marrow. Hematopoietic differentiation is finely tuned by cell-intrinsic mechanisms and lineage-specific transcription factors. However, it is now clear that the bone marrow microenvironment plays an essential role in the maintenance of hematopoietic stem cells (HSC) and their differentiation into more mature lineages. Mesenchymal and endothelial cells contribute to a protective microenvironment called hematopoietic niches that secrete specific factors and establish a direct contact with developing hematopoietic cells. A number of recent studies have addressed in mouse models the specific molecular events that are involved in the cellular crosstalk between hematopoietic subsets and their niches. This has led to the concept that hematopoietic differentiation and commitment towards a given hematopoietic pathway is a dynamic process controlled at least partially by the bone marrow microenvironment. In this review, we discuss the evolving view of murine hematopoietic⁻stromal cell crosstalk that is involved in HSC maintenance and commitment towards B cell differentiation.
Keywords: B lymphopoiesis; bone marrow niches; early hematopoiesis; stromal cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

