Synthesis of 2,6-Diamino-Substituted Purine Derivatives and Evaluation of Cell Cycle Arrest in Breast and Colorectal Cancer Cells
- PMID: 30103421
- PMCID: PMC6222518
- DOI: 10.3390/molecules23081996
Synthesis of 2,6-Diamino-Substituted Purine Derivatives and Evaluation of Cell Cycle Arrest in Breast and Colorectal Cancer Cells
Abstract
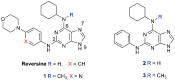
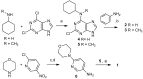
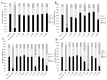
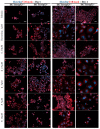
Reversine is a potent antitumor 2,6-diamino-substituted purine acting as an Aurora kinases inhibitor and interfering with cancer cell cycle progression. In this study we describe three reversine-related molecules, designed by docking calculation, that present structural modifications in the diamino units at positions 2 and 6. We investigated the conformations of the most stable prototropic tautomers of one of these molecules, the N6-cyclohexyl-N6-methyl-N2-phenyl-7H-purine-2,6-diamine (3), by Density Functional Theory (DFT) calculation in the gas phase, water and chloroform, the last solvent considered to give insights into the detection of broad signals in NMR analysis. In all cases the HN(9) tautomer resulted more stable than the HN(7) form, but the most stable conformations changed in different solvents. Molecules 1⁻3 were evaluated on MCF-7 breast and HCT116 colorectal cancer cell lines showing that, while being less cytotoxic than reversine, they still caused cell cycle arrest in G2/M phase and polyploidy. Unlike reversine, which produced a pronounced cell cycle arrest in G2/M phase in all the cell lines used, similar concentrations of 1⁻3 were effective only in cells where p53 was deleted or down-regulated. Therefore, our findings support a potential selective role of these structurally simplified, reversine-related molecules in p53-defective cancer cells.
Keywords: cell cycle arrest; endoreduplication; microwave-assisted synthesis; molecular docking; p53; reversine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Anastasia L., Sampaolesi M., Papini N., Oleari D., Lamorte G., Tringali C., Monti E., Galli D., Tettamanti G., Cossu G., et al. Reversine-treated fibroblasts acquire myogenic competence in vitro and in regenerating skeletal muscle. Cell Death Differ. 2006;13:2042–2051. doi: 10.1038/sj.cdd.4401958. - DOI - PubMed
-
- Pikir B.S., Susilowati H., Hendrianto E., Abdulrantam F. Reversine increase the plasticity of bone marrow-derived mesenchymal stem cell for generation of cardiomyocyte in vitro. Acta Med. Indones. 2012;44:23–27. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

