Influenza Virus Infection of Human Lymphocytes Occurs in the Immune Cell Cluster of the Developing Antiviral Response
- PMID: 30103427
- PMCID: PMC6115886
- DOI: 10.3390/v10080420
Influenza Virus Infection of Human Lymphocytes Occurs in the Immune Cell Cluster of the Developing Antiviral Response
Abstract
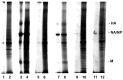
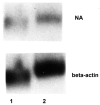
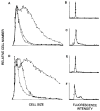
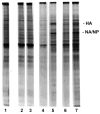
Monocytes-macrophages and lymphocytes are recruited to the respiratory tract in response to influenza virus challenge and are exposed to the virus during the establishment of immune defenses. The susceptibility of human lymphocytes to infection was assessed. The presence of monocytes-macrophages was required to attain infection of both resting and proliferating lymphocytes. Lymphocyte infection occurred in the context of immune cell clusters and was blocked by the addition of anti-intercellular adhesion molecule-1 (ICAM-1) antibody to prevent cell clustering. Both peripheral blood-derived and bronchoalveolar lymphocytes were susceptible to infection. Both CD4⁺ and CD8⁺ T lymphocytes were susceptible to influenza virus infection, and the infected CD4⁺ and CD8⁺ lymphocytes served as infectious foci for other nonpermissive or even virus-permissive cells. These data show that monocytes-macrophages and both CD4⁺ and CD8⁺ lymphocytes can become infected during the course of an immune response to influenza virus challenge. The described leukocyte interactions during infection may play an important role in the development of effective anti-influenza responses.
Keywords: alveolar lymphocytes; human lymphocytes; human macrophages; human monocytes; immune cell clusters; influenza virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Cerwenka A., Morgan T.M., Dutton R.W. Naive, Effector, and Memory CD8 T Cells in Protection Against Pulmonary Influenza Virus Infection: Homing Properties Rather Than Initial Frequencies Are Crucial. J. Immunol. 1999;163:5535–5543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

