Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates
- PMID: 30103434
- PMCID: PMC6222762
- DOI: 10.3390/molecules23081997
Chemical Synthesis of Rare, Deoxy-Amino Sugars Containing Bacterial Glycoconjugates as Potential Vaccine Candidates
Abstract
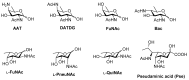
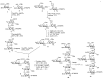
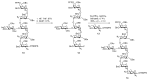
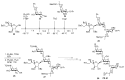
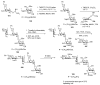
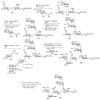
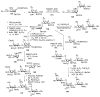
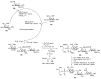
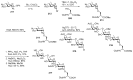
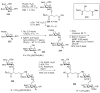
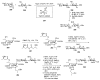
Bacteria often contain rare deoxy amino sugars which are absent in the host cells. This structural difference can be harnessed for the development of vaccines. Over the last fifteen years, remarkable progress has been made toward the development of novel and efficient protocols for obtaining the rare sugar building blocks and their stereoselective assembly to construct conjugation ready bacterial glycans. In this review, we discuss the total synthesis of a variety of rare sugar containing bacterial glycoconjugates which are potential vaccine candidates.
Keywords: bacterial glycoconjugates; rare deoxy-amino sugars; stereoselective glycosylation; total synthesis; vaccine candidates; zwitterionic polysaccharides.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

















References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

