Membrane-Associated Proteins in Giardia lamblia
- PMID: 30103435
- PMCID: PMC6115752
- DOI: 10.3390/genes9080404
Membrane-Associated Proteins in Giardia lamblia
Abstract
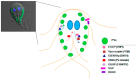
The manner in which membrane-associated proteins interact with the membrane defines their subcellular fate and function. This interaction relies on the characteristics of the proteins, their journey after synthesis, and their interaction with other proteins or enzymes. Understanding these properties may help to define the function of a protein and also the role of an organelle. In the case of microorganisms like protozoa parasites, it may help to understand singular features that will eventually lead to the design of parasite-specific drugs. The protozoa parasite Giardia lamblia is an example of a widespread parasite that has been infecting humans and animals from ancestral times, adjusting itself to the changes of the environment inside and outside the host. Several membrane-associated proteins have been posted in the genome database GiardiaDB, although only a few of them have been characterized. This review discusses the data regarding membrane-associated proteins in relationship with lipids and specific organelles and their implication in the discovery of anti-giardial therapies.
Keywords: PX domain; lysosomal vacuoles; palmitoylation; phosphoinositides; variant-specific surface protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Adl S.M., Simpson A.G., Farmer M.A., Andersen R.A., Anderson O.R., Barta J.R., Bowser S.S., Brugerolle G., Fensome R.A., Fredericq S., et al. The new higher level classification of eukaryotes with emphasis on the taxonomy of protists. J. Eukaryot. Microbiol. 2005;52:399–451. doi: 10.1111/j.1550-7408.2005.00053.x. - DOI - PubMed
-
- Soltys B.J., Falah M., Gupta R.S. Identification of endoplasmic reticulum in the primitive eukaryote Giardia lamblia using cryoelectron microscopy and antibody to bip. Pt 7J. Cell Sci. 1996;109:1909–1917. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

