Early Investigations and Recent Advances in Intraperitoneal Immunotherapy for Peritoneal Metastasis
- PMID: 30103457
- PMCID: PMC6160982
- DOI: 10.3390/vaccines6030054
Early Investigations and Recent Advances in Intraperitoneal Immunotherapy for Peritoneal Metastasis
Erratum in
-
Erratum: Thadi A.; et al. Early Investigations and Recent Advances in Intraperitoneal Immunotherapy for Peritoneal Metastasis. Vaccines 2018, 6, 54.Vaccines (Basel). 2019 Jan 31;7(1):15. doi: 10.3390/vaccines7010015. Vaccines (Basel). 2019. PMID: 30708956 Free PMC article.
Abstract
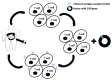
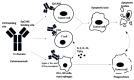
Peritoneal metastasis (PM) is an advanced stage malignancy largely refractory to modern therapy. Intraperitoneal (IP) immunotherapy offers a novel approach for the control of regional disease of the peritoneal cavity by breaking immune tolerance. These strategies include heightening T-cell response and vaccine induction of anti-cancer memory against tumor-associated antigens. Early investigations with chimeric antigen receptor T cells (CAR-T cells), vaccine-based therapies, dendritic cells (DCs) in combination with pro-inflammatory cytokines and natural killer cells (NKs), adoptive cell transfer, and immune checkpoint inhibitors represent significant advances in the treatment of PM. IP delivery of CAR-T cells has shown demonstrable suppression of tumors expressing carcinoembryonic antigen. This response was enhanced when IP injected CAR-T cells were combined with anti-PD-L1 or anti-Gr1. Similarly, CAR-T cells against folate receptor α expressing tumors improved T-cell tumor localization and survival when combined with CD137 co-stimulatory signaling. Moreover, IP immunotherapy with catumaxomab, a trifunctional antibody approved in Europe, targets epithelial cell adhesion molecule (EpCAM) and has shown considerable promise with control of malignant ascites. Herein, we discuss immunologic approaches under investigation for treatment of PM.
Keywords: CAR-T cells; ascites; carcinoembryonic antigen; dendritic cells; folate receptor α; intraperitoneal immunotherapy; metastasis; peritoneal; vaccines.
Conflict of interest statement
The author declares no conflict of interest.
Figures


References
-
- Kerscher A.G., Chua T.C., Gasser M., Maeder U., Kunzmann V., Isbert C., Germer C.T., Pelz J.O. Impact of peritoneal carcinomatosis in the disease history of colorectal cancer management: A longitudinal experience of 2406 patients over two decades. Br. J. Cancer. 2013;108:1432–1439. doi: 10.1038/bjc.2013.82. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

