The effect of intracerebroventricular administration of orexin receptor type 2 antagonist on pentylenetetrazol-induced kindled seizures and anxiety in rats
- PMID: 30103703
- PMCID: PMC6090721
- DOI: 10.1186/s12868-018-0445-9
The effect of intracerebroventricular administration of orexin receptor type 2 antagonist on pentylenetetrazol-induced kindled seizures and anxiety in rats
Abstract
Background: Current antiepileptic drugs are not able to prevent recurrent seizures in all patients. Orexins are excitatory hypothalamic neuropeptides that their receptors (Orx1R and Orx2R) are found almost in all major regions of the brain. Pentylenetetrazol (PTZ)-induced kindling is a known experimental model for epileptic seizures. The purpose of this study was to evaluate the effect of Orx2 receptor antagonist (TCS OX2 29) on seizures and anxiety of PTZ-kindled rats.
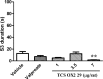
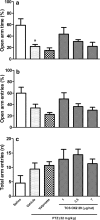
Results: Our results revealed that similar to valproate, administration of 7 µg/rat of TCS OX2 29 increased the latency period and decreased the duration time of 3rd and 4th stages of epileptiform seizures. Besides, it significantly decreased mean of seizure scores. However, TCS OX2 29 did not modulate anxiety induced by repeated PTZ administration.
Conclusion: This study showed that blockade of Orx2 receptor reduced seizure-related behaviors without any significant effect on PTZ-induced anxiety.
Keywords: Anxiety; Kindling; Orexin; Orx2 receptor; PTZ; Seizure.
Figures




Similar articles
-
SB-334867, an orexin receptor 1 antagonist, decreased seizure and anxiety in pentylenetetrazol-kindled rats.Fundam Clin Pharmacol. 2017 Apr;31(2):201-207. doi: 10.1111/fcp.12249. Epub 2016 Nov 23. Fundam Clin Pharmacol. 2017. PMID: 27739093
-
Comparison between standard protocol and a novel window protocol for induction of pentylenetetrazol kindled seizures in the rat.Epilepsy Res. 2013 Sep;106(1-2):54-63. doi: 10.1016/j.eplepsyres.2013.03.016. Epub 2013 Apr 22. Epilepsy Res. 2013. PMID: 23619005
-
Hippocampal orexin receptors inactivation reduces PTZ induced seizures of male rats.Pharmacol Biochem Behav. 2015 Mar;130:77-83. doi: 10.1016/j.pbb.2015.01.006. Epub 2015 Jan 17. Pharmacol Biochem Behav. 2015. PMID: 25600753
-
[Impaired memory following repeated pentylenetetrazol treatments in kindled mice].Yakugaku Zasshi. 2012;132(2):179-82. doi: 10.1248/yakushi.132.179. Yakugaku Zasshi. 2012. PMID: 22293696 Review. Japanese.
-
Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: Potential for therapy.Brain Res. 2020 Mar 15;1731:146085. doi: 10.1016/j.brainres.2018.12.036. Epub 2018 Dec 24. Brain Res. 2020. PMID: 30590027 Free PMC article. Review.
Cited by
-
Decreased Cerebrospinal Fluid Orexin-A (Hypocretin-1) Concentrations in Patients after Generalized Convulsive Status Epilepticus.J Clin Med. 2020 Oct 19;9(10):3354. doi: 10.3390/jcm9103354. J Clin Med. 2020. PMID: 33086714 Free PMC article.
-
Self-Assembled Lecithin-Chitosan Nanoparticles Improved Rotigotine Nose-to-Brain Delivery and Brain Targeting Efficiency.Pharmaceutics. 2023 Mar 5;15(3):851. doi: 10.3390/pharmaceutics15030851. Pharmaceutics. 2023. PMID: 36986712 Free PMC article.
-
Dual orexin antagonist normalized sleep homeostatic drive, enhanced GABAergic inhibition, and suppressed seizures after traumatic brain injury.Sleep. 2022 Dec 12;45(12):zsac238. doi: 10.1093/sleep/zsac238. Sleep. 2022. PMID: 36165953 Free PMC article.
-
Bypassing the Blood-Brain Barrier: Direct Intracranial Drug Delivery in Epilepsies.Pharmaceutics. 2020 Nov 24;12(12):1134. doi: 10.3390/pharmaceutics12121134. Pharmaceutics. 2020. PMID: 33255396 Free PMC article. Review.
-
Sudden Unexpected Death in Epilepsy: A Narrative Review of Mechanism, Risks, and Prevention.J Clin Med. 2025 May 10;14(10):3329. doi: 10.3390/jcm14103329. J Clin Med. 2025. PMID: 40429323 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

