RAS modulation prevents progressive cognitive impairment after experimental stroke: a randomized, blinded preclinical trial
- PMID: 30103772
- PMCID: PMC6090822
- DOI: 10.1186/s12974-018-1262-x
RAS modulation prevents progressive cognitive impairment after experimental stroke: a randomized, blinded preclinical trial
Abstract
Background: With the aging population, the prevalence and incidence of cerebrovascular disease will continue to rise, as well as the number of individuals with vascular cognitive impairment/dementia (VCID). No specific FDA-approved treatments for VCID exist. Although clinical evidence supports that angiotensin receptor blockers (ARBs) prevent cognitive decline in older adults, whether ARBs have a similar effect on VCID after stroke is unknown. Moreover, these agents reduce BP, which is undesirable in the acute stroke period, so we believe that giving C21 in this acute phase or delaying ARB administration would enable us to achieve the neurovascular benefits without the risk of unintended and potentially dangerous, acute BP lowering.
Methods: The aim of our study was to determine the impact of candesartan (ARB) or compound-21 (an angiotensin type 2 receptor--AT2R--agonist) on long-term cognitive function post-stroke, in spontaneously hypertensive rats (SHRs). We hypothesized that AT2R stimulation, either directly with C21, or indirectly by blocking the angiotensin type 1 receptor (AT1R) with candesartan, initiated after stroke, would reduce cognitive impairment. Animals were subjected to a 60-min transient middle cerebral artery occlusion and randomly assigned to either saline/C21 monotherapy, for the full study duration (30 days), or given sequential therapy starting with saline/C21 (7 days) followed by candesartan for the remainder of the study (21 days). Outcome measures included sensorimotor/cognitive-function, amyloid-β determination, and histopathologic analyses.
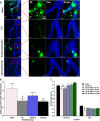
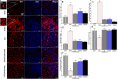
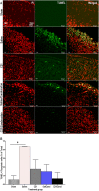
Results: Treatment with RAS modulators effectively preserved cognitive function, reduced cytotoxicity, and prevented chronic-reactive microgliosis in SHRs, post-stroke. These protective effects were apparent even when treatment was delayed up to 7 days post-stroke and were independent of blood pressure and β-amyloid accumulation.
Conclusion: Collectively, our findings demonstrate that RAS modulators effectively prevent cognitive impairment after stroke, even when treatment is delayed.
Keywords: Angiotensin modulators; Cognitive-impairment; Hypertension; Stroke.
Conflict of interest statement
All experimental procedures and animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of Charlie Norwood VA Medical Center, Augusta, GA, USA.
Not applicable
Drugs were supplied from Astra-Zeneca and Vicore Pharma. The authors declared that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Rouch L, Cestac P, Hanon O, Cool C, Helmer C, Bouhanick B, et al. Antihypertensive drugs, prevention of cognitive decline and dementia: a systematic review of observational studies, randomized controlled trials and meta-analyses, with discussion of potential mechanisms. CNS Drugs. 2015;29:113–130. doi: 10.1007/s40263-015-0230-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

