Impact of monovalent rotavirus vaccine on diarrhoea-associated post-neonatal infant mortality in rural communities in Malawi: a population-based birth cohort study
- PMID: 30103981
- PMCID: PMC6088152
- DOI: 10.1016/S2214-109X(18)30314-0
Impact of monovalent rotavirus vaccine on diarrhoea-associated post-neonatal infant mortality in rural communities in Malawi: a population-based birth cohort study
Erratum in
-
Correction to Lancet Glob Health 2018; 6: e1036-44.Lancet Glob Health. 2018 Dec;6(12):e1286. doi: 10.1016/S2214-109X(18)30402-9. Epub 2018 Aug 22. Lancet Glob Health. 2018. PMID: 30143444 Free PMC article. No abstract available.
Abstract
Background: Rotavirus is a major contributor to child mortality. The effect of rotavirus vaccine on diarrhoea mortality has been estimated in middle-income but not low-income settings, where mortality is high and vaccine effectiveness in reducing admissions to hospital is lower. Empirical population-based mortality studies have not been done in any setting. Malawi introduced monovalent rotavirus vaccine (RV1) in October, 2012. We aimed to investigate the impact and effectiveness of the RV1 vaccine in reducing diarrhoea-associated mortality in infants aged 10-51 weeks.
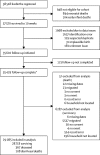
Methods: In this population-based cohort study, we included infants born between Jan 1, 2012, and June 1, 2015, in Mchinji, Central Malawi and analysed data on those surviving 10 weeks. Individual vaccination status was extracted from caregiver-held records or report at home visits at 4 months and 1 year of age. Survival to 1 year was confirmed at home visit, or cause of death ascertained by verbal autopsy. We assessed impact (1 minus mortality rate ratio following vs before vaccine introduction) using Poisson regression. Among vaccine-eligible infants (born from Sept 17, 2012), we assessed effectiveness (1 minus hazard ratio) using Cox regression.
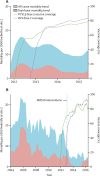
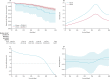
Findings: Between Jan 1, 2012, and June 1, 2015, we recruited 48 672 livebirths in Mchinji, among whom 38 518 were vaccine-eligible and 37 570 survived to age 10 weeks. Two-dose versus zero-dose effectiveness analysis included 28 141 infants, of whom 101 had diarrhoea-associated death before 1 year of age. Diarrhoea-associated mortality declined by 31% (95% CI 1-52; p=0·04) after RV1 introduction. Effectiveness against diarrhoea-mortality was 34% (95% CI -28 to 66; p=0·22).
Interpretation: RV1 was associated with substantial reduction in diarrhoea-associated deaths among infants in this rural sub-Saharan African setting. These data add considerable weight to evidence showing the impact of rotavirus vaccine programmes.
Funding: Wellcome Trust and GlaxoSmithKline Biologicals.
Copyright © 2018 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY-NC-ND 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures
Comment in
-
Rotavirus vaccine impact in Africa: greater than the sum of its parts?Lancet Glob Health. 2018 Sep;6(9):e948-e949. doi: 10.1016/S2214-109X(18)30356-5. Lancet Glob Health. 2018. PMID: 30103987 No abstract available.
References
-
- WHO Global health observatory data. Causes of child mortality. 2016. http://www.who.int/gho/child_health/mortality/causes/en/
-
- ROTA Council (Rotavirus Organization of Technical Allies) Global introduction status. National and regional rotavirus vaccine introductions. http://rotacouncil.org/vaccine-introduction/global-introduction-status/
-
- Groome MJ, Page N, Cortese MM. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in South African children: a case-control study. Lancet Infect Dis. 2014;14:1096–1104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

